Radioactivity Explained
3. Alpha, beta and gamma
As simple as the abc
3.1 Becquerel discovers radiation from uranium
3.2 Marie Curie finds more radioactive elements
3.3 Rutherford separates alpha, beta and gamma
3.4 The sparkler analogy
3.5 Nuclear changes with alpha, beta, gamma
3.6 Penetration and ionisation
3.7 Detecting radiation
3.1 Becquerel discovers radiation from uranium
Short video summary (1:47)
-
Henri Becquerel discovered by accident that uranium gave off a strange kind of unknown radiation
-
In 1896 the French physicist, Henri Becquerel was experimenting with rocks containing uranium. He was interested in the way they glowed in sunlight but didn't when it was cloudy - an effect called fluorescence.
Crookes tubes (see Lesson 2) had just been discovered to give off X-rays if you used a high enough voltage, and Becquerel wanted know whether uranium rocks gave off X-rays when they fluoresced.
Scientists knew that X-rays fogged film. Becquerel covered some unexposed film with a light-proof lid, placed a thin metal shape on top of the lid and the uranium on top of that. He left it for a while, and when he developed the film found that it had been fogged.
The shape left by the metal shape showed that whatever was fogging the film couldn't go through metal. This was pretty good evidence that X-rays were also given off when the uranium was fluorescing.
When he came to repeat the experiment the day was cloudy so there was no fluorescence. So he set the experiment up, put it in a draw, and waited for it get sunny again.
After a day or so there was still no sun so he decided to develop the film anyway. Surprisingly the film was fogged just as much as before.
After more experiments Becquerel concluded that uranium gave off strange new rays all the time, whether it was fluorescing or not. Becquerel called them uranium rays - but we now know they were a type of nuclear radiation made up of little particles.
-
Even though this is often portrayed as a piece of luck, it is almost certain that Becquerel would soon have discovered that uranium always fogged film, regardless of whether it was fluorescing at the same time.
It’s a good story, though, and you could discuss what else Becquerel could have done but develop the film. If he didn’t, would it have been valid to use it in another experiment?
Key animations from the video for you to use in front of a class
Did fluorescence cause X-rays?
Becquerel investigates fluorescence
Roentgen discovers X-rays
No - uranium always fogged film
Becquerel discovers radioactivity
3.2 Marie Curie finds more radioactive elements
Short video summary (3:12)
-
Marie Curie investigated radioactivity with Henri Becquerel and her husband, Pierre
She discovered other radioactive elements that occurred naturally with uranium
She is the only person to have been awarded two Nobel Prizes in different subjects - physics and chemistry
-
Marie Curie was a Polish scientist living and working in Paris. She and Henri Becquerel laid the foundations for understanding radioactivity - which was the word Marie Curie came up with.
At the time everyone thought radiation was made up of waves, even though we now know it's mostly particles.
One big question was how did uranium manage to radiate all the time? When you burn a match, there's a chemical reaction between the wood and the air, and the match eventually burns out. This didn't seem to happen with uranium - it didn't seem to be running out of anything
No matter what chemical reactions the uranium underwent, the amount of radiation never changed. Its radioactivity only seemed to depend on how much there was, not what it was combined with.
This suggested that it was something about the atoms of uranium themselves that was producing the radiation. Marie Curie's research was happening before Rutherford had deduced that atoms had a nucleus - so she had no good model for what might be the source of the radiation.
Marie Curie, her husband, Pierre Curie, and Henri Becquerel were awarded the Nobel prize for Physics in 1903 for their work on radioactivity.
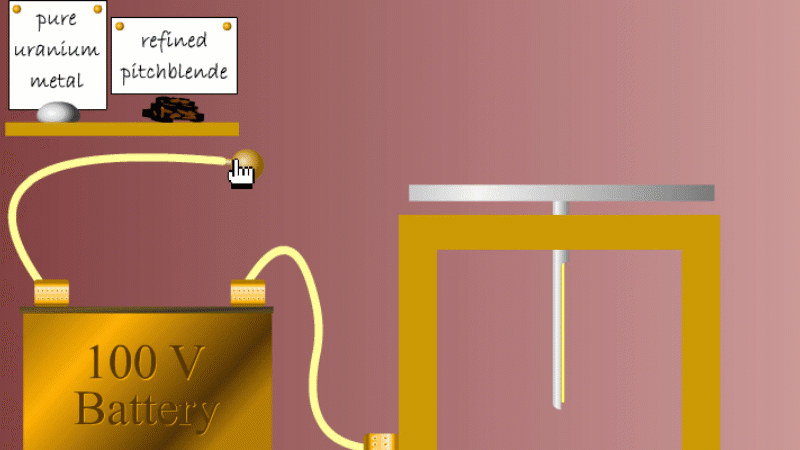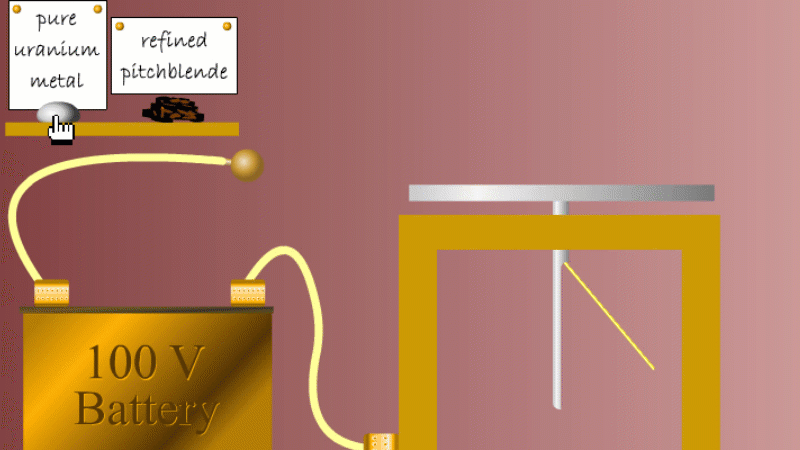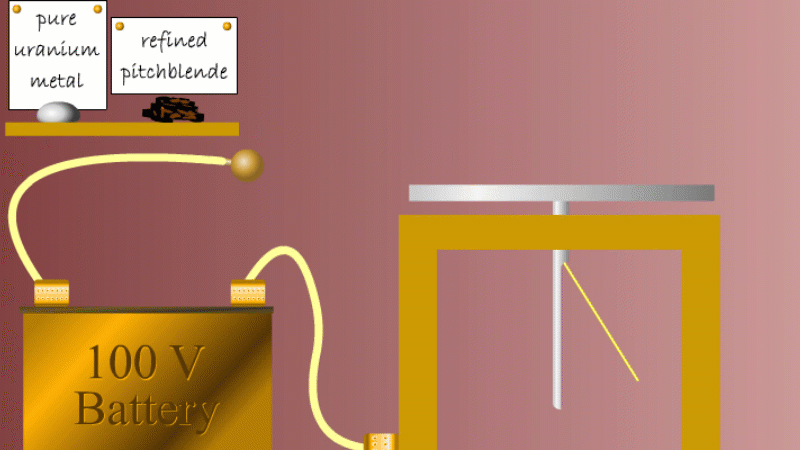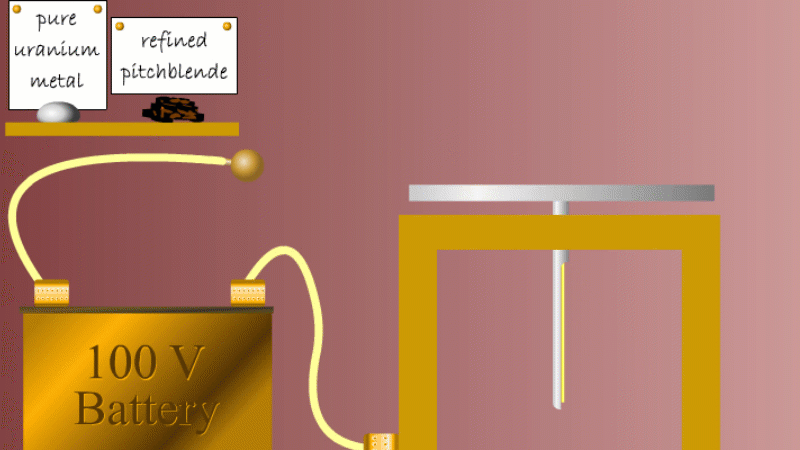
Marie and Pierre continued to search for other radioactive elements. They found that pure uranium metal was actually less radioactive than the ore in which it was found - called pitchblende.
Marie Curie reasoned that this must be because there were other radioactive elements in the pitchblende that hadn't been discovered because they were only there in minute quantities. And to make such a big difference to the overall radioactivity these trace elements must be highly radioactive.
Marie and Pierre started with several tonnes of pitchblende and after three years developing advanced refinement techniques they managed to isolate less than a gram each of two new highly radioactive elements that were naturally present in the pitchblende. One she called polonium after her native Poland, and the other radium because of the amount of radiation it gave off.
Pierre was killed in a road accident in 1906, so it was Marie alone who was awarded the Nobel Prize for Chemistry in 1911. She is the only woman to have won two Nobel Prizes, and the only person to have been awarded a prize for different disciplines - physics and chemistry.
Radium became important for the development of physics because it could produce a concentrated beam of radiation that could be used in experiments.
Marie spent the rest of her life promoting medical treatments based on radiation.
-
Once the Curies had isolated radium, they became for a short while the only people in the world who had any. There was huge demand from other scientists all over the world, including Rutherford - who was working at a university in Canada at the time.
We’ll see later on that uranium and polonium are decay products of uranium - in other words uranium gradually turns into other elements when the nuclei of uranium atoms change, and emit radiation.
Key animations from the video for you to use in front of a class
Marie Curie predicts new elements
Radioactivity only depended on amount
The ore is more radioactive than the metal!
Marie Curie isolates radium
3.3 Rutherford separates alpha, beta and gamma
Short video summary (3:15)
-
Ernest Rutherford discovered that radiation consisted of two types of particle by how they were blocked by different thickness of very thin metal foils
He called the two types alpha and beta radiation
He named gamma radiation, but didn’t discover it
-
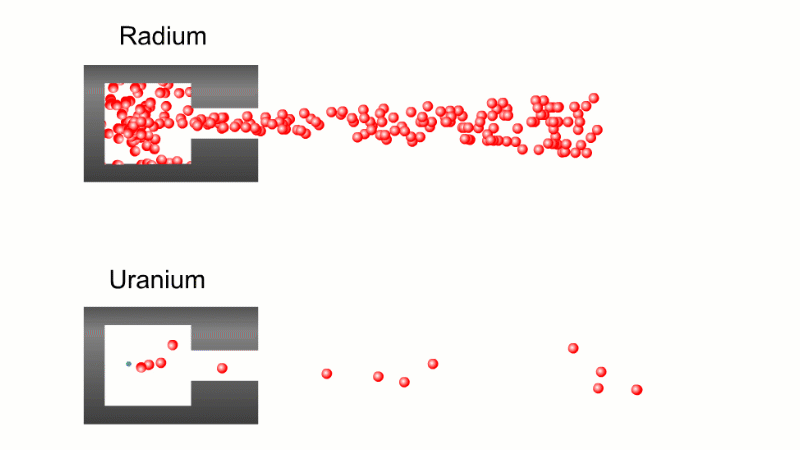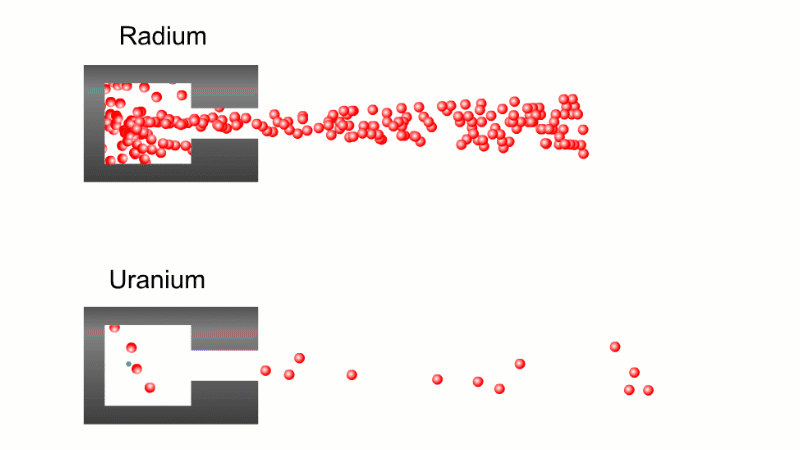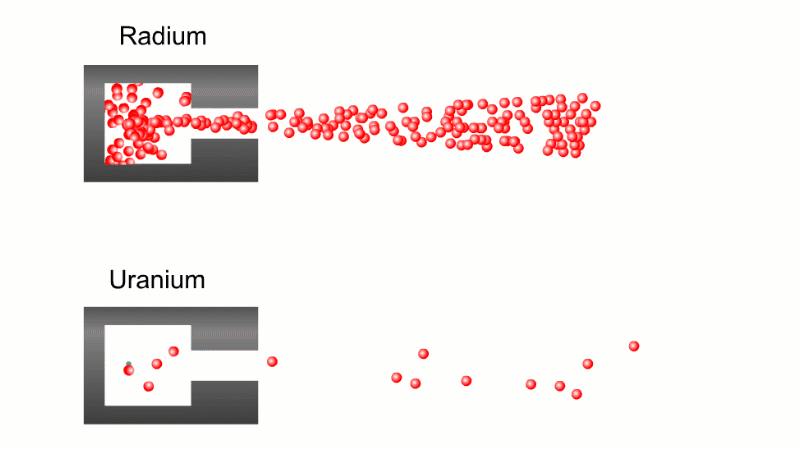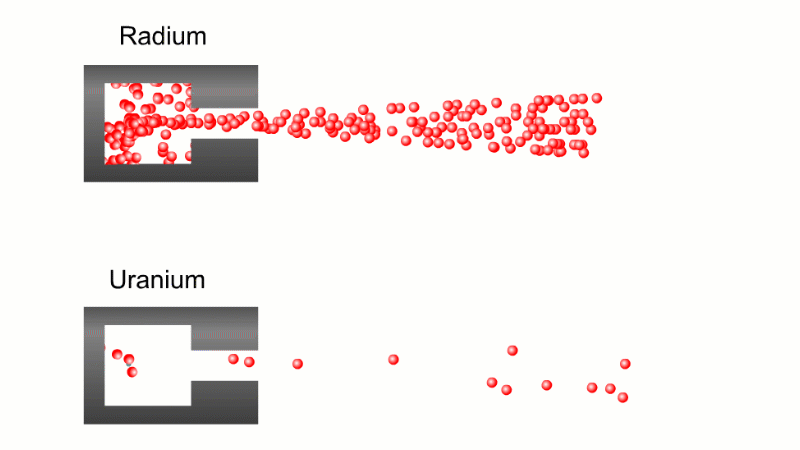
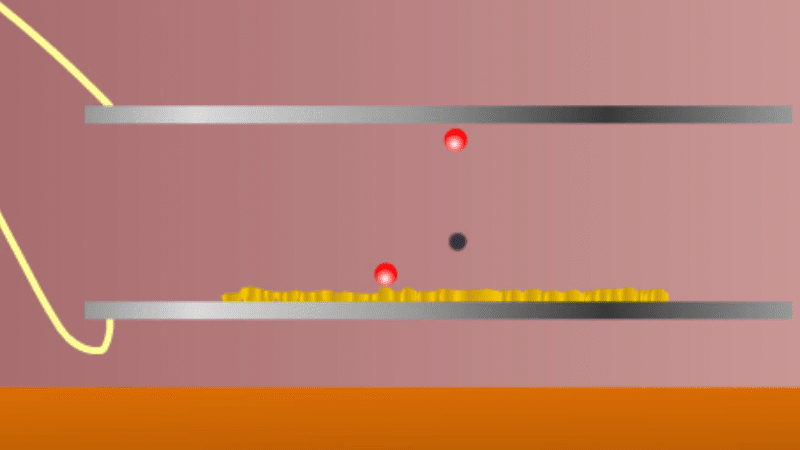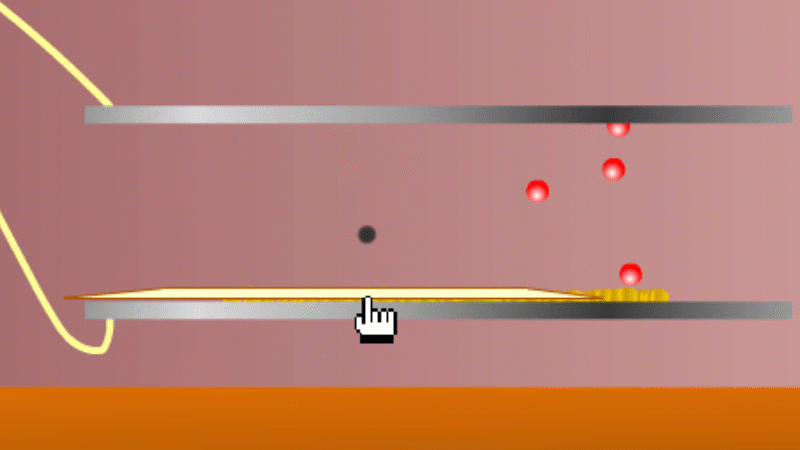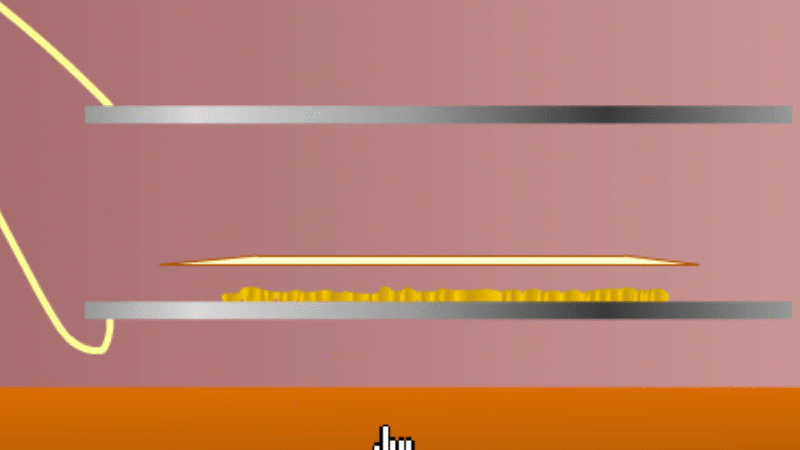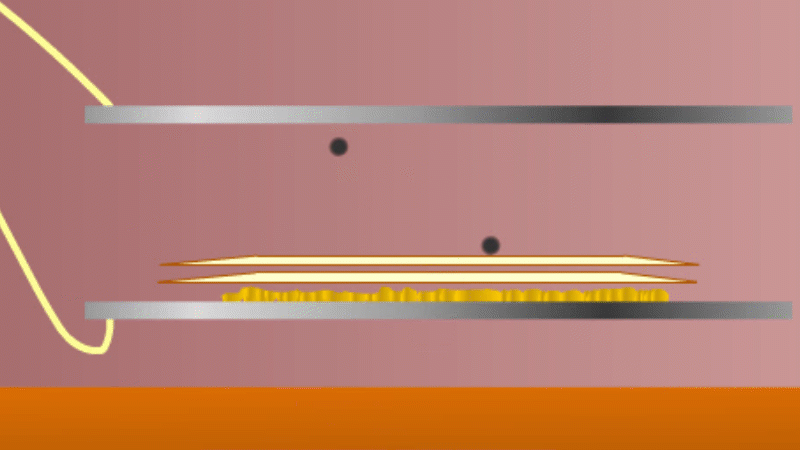
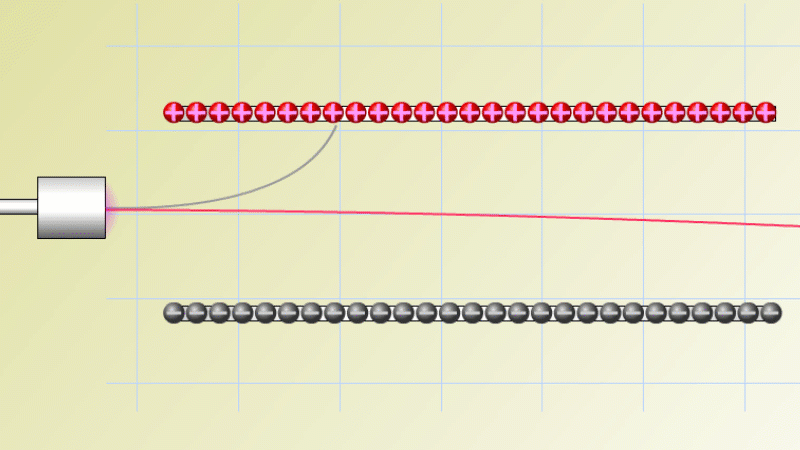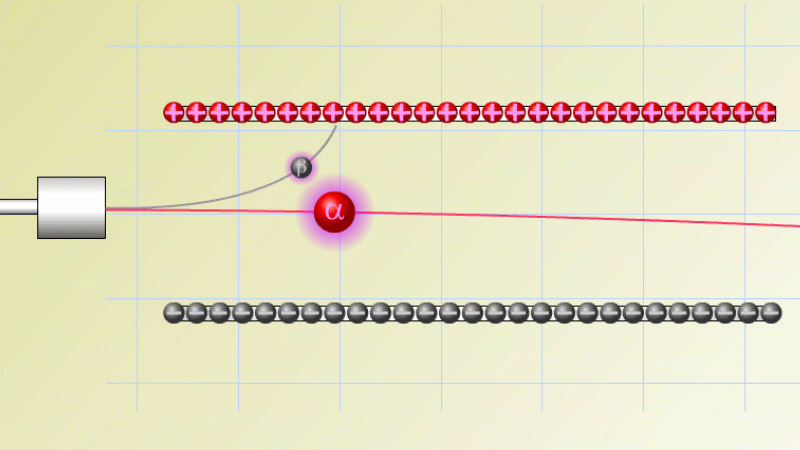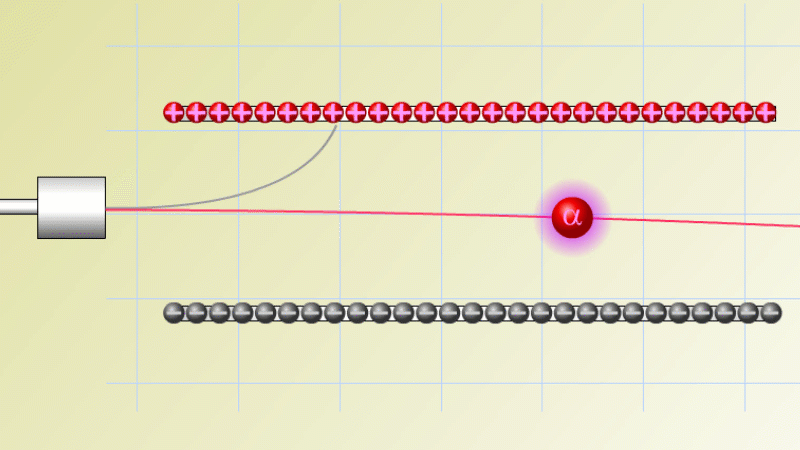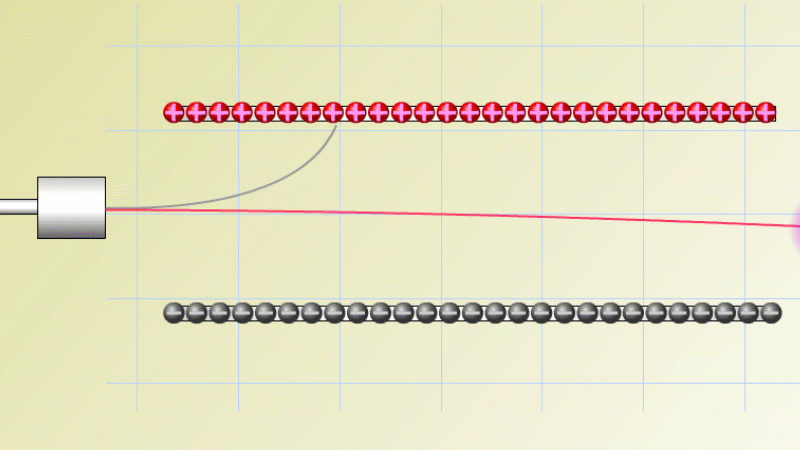
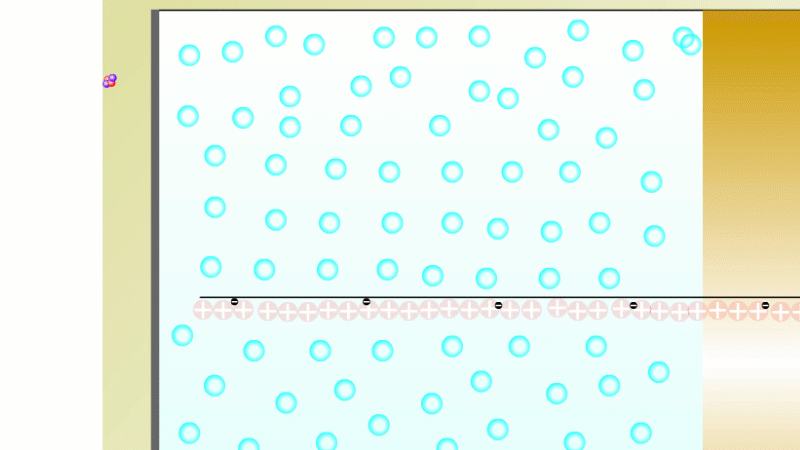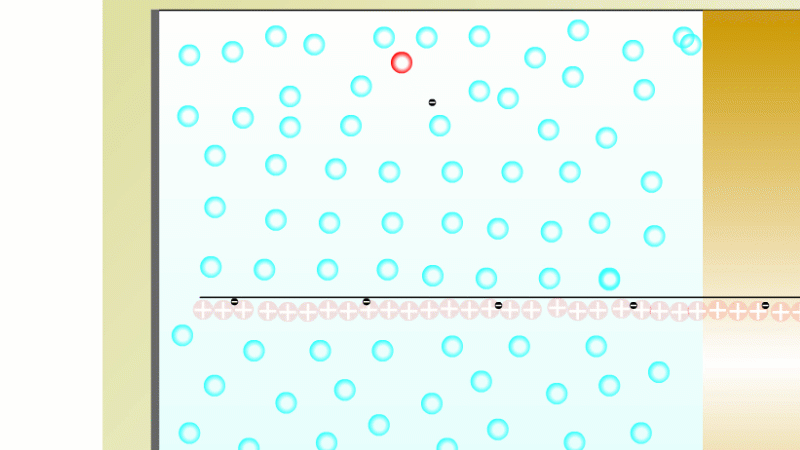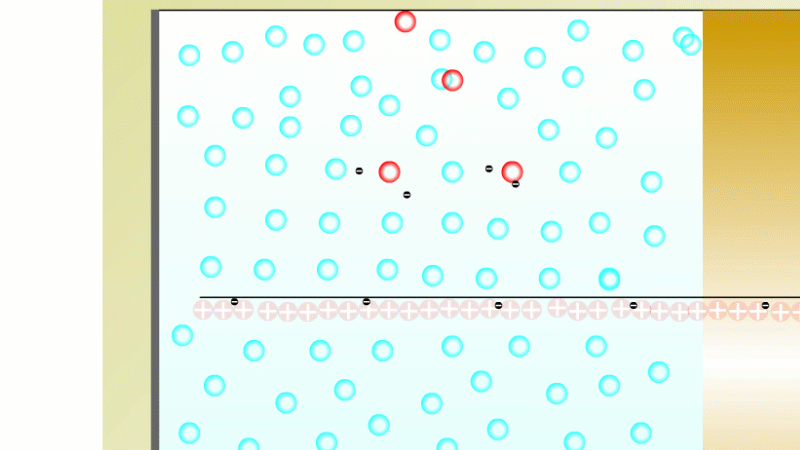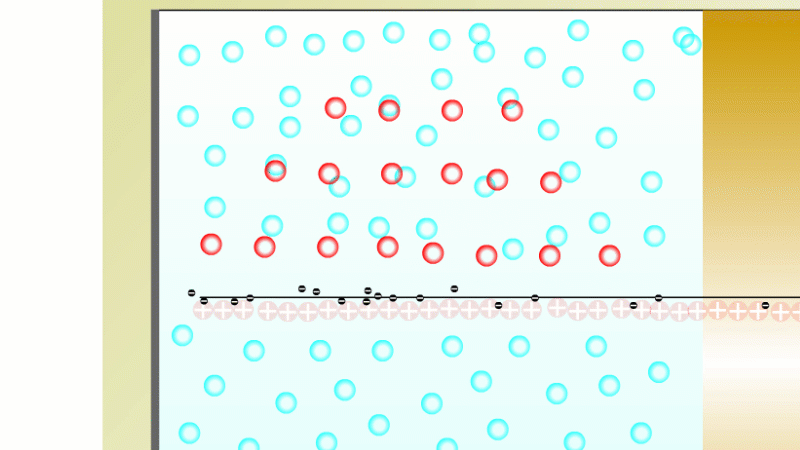
In 1899 Rutherford was experimenting with how easily the radiation from powdered uranium went through very, very thin sheets of metal foil. In one detector the more radiation that's detected, the faster a light moved across a screen.
With no sheets present the light went pretty quickly. When you added a single sheet it slowed down a lot. You'd expect it to slow to almost nothing if you added a second sheet. But in fact it made almost no difference. That was very puzzling.
Rutherford's conclusion was that the radiation consisted of particles, not waves, as most scientists thought. And what's more there were two types of particle.
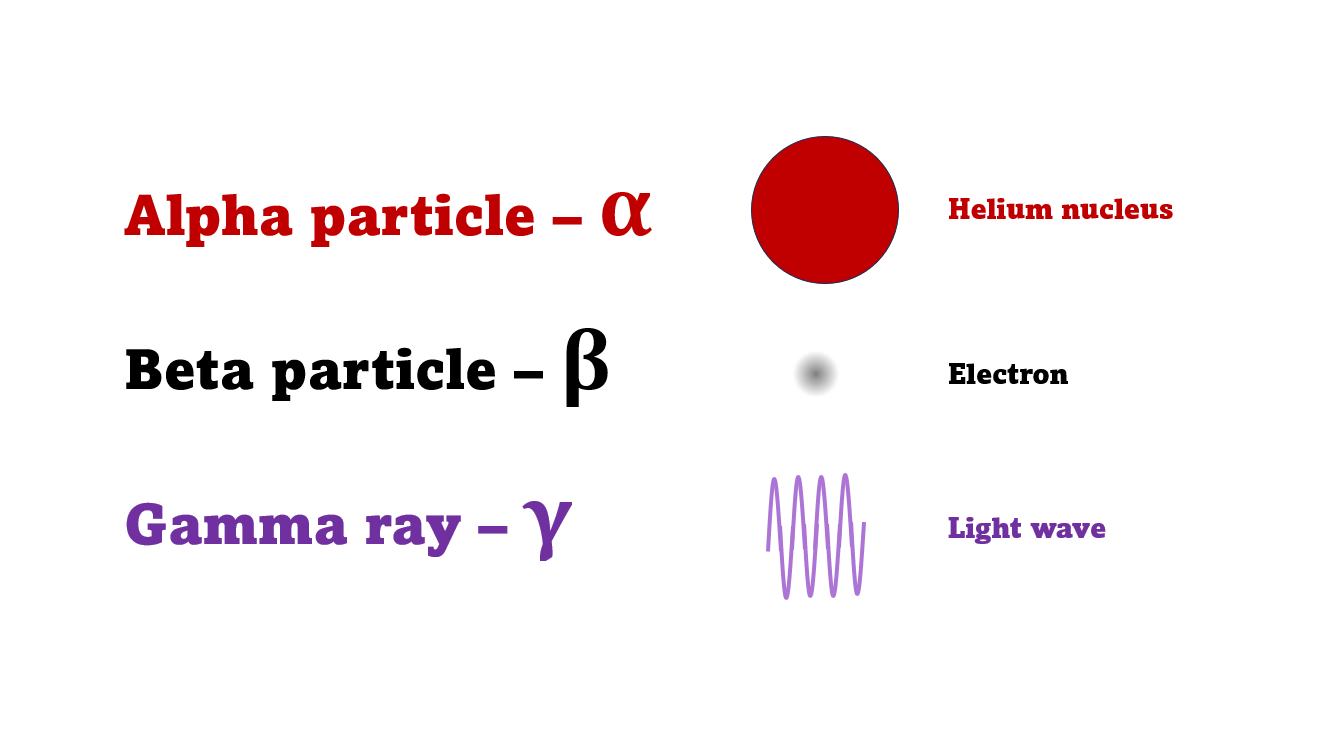
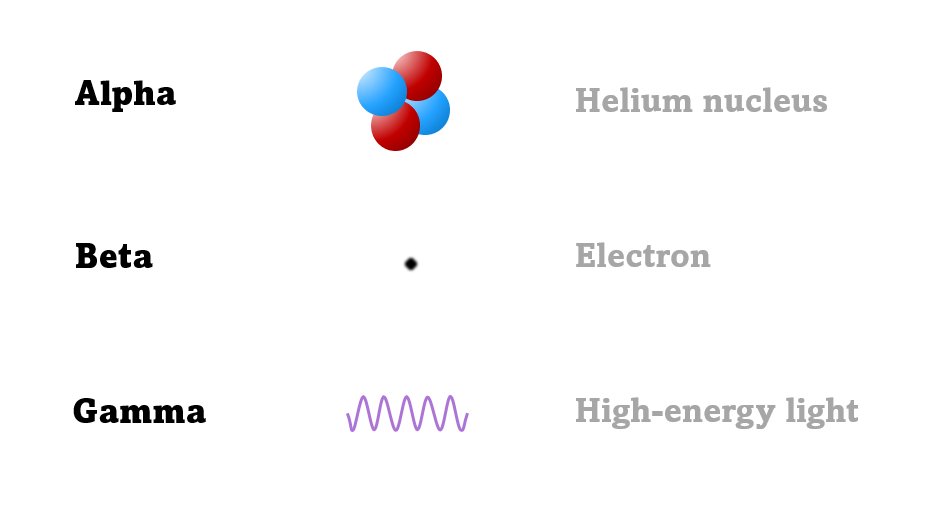
The first type was stopped by a single sheet - Rutherford called these alpha particles. The second passed easily through two sheets - Rutherford called these beta particles. He was just using the Greek version of the letters A and B.
By measuring how alpha and beta particles moved between electrically charged plates Rutherford could tell that alpha particles were positively charged, with a big mass, and beta particles were negatively charged with a very small mass.
In fact beta particles behaved in exactly the same way as electrons (spoiler alert - they are electrons, but as we'll see they come from changes in the nucleus not from the outer electrons of the atom).
Alpha particles seemed to behave like a helium atom with a double positive charge (we know it's actually a helium nucleus, but Rutherford hadn't discovered the nucleus yet!).
All the early discoveries about radioactivity were made before scientists had developed our modern picture of the atom - one with a nucleus at its centre made of protons and neutrons. In fact scientists could only investigate the structure of the atom using the radiation it produced - so it was a bit of a chicken and egg situation.
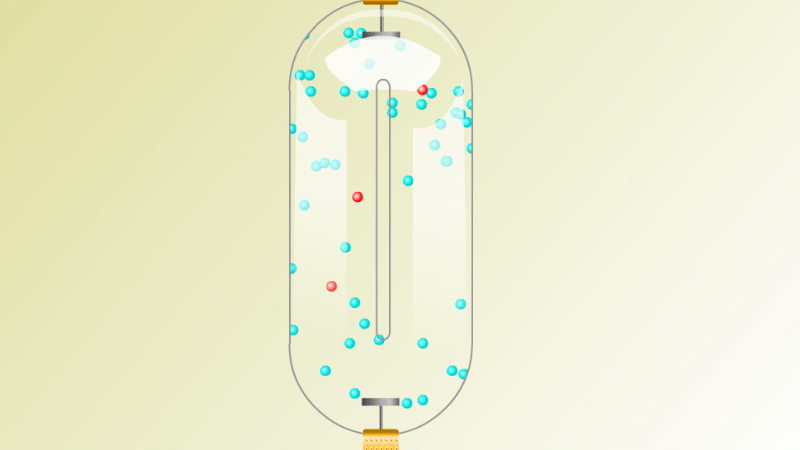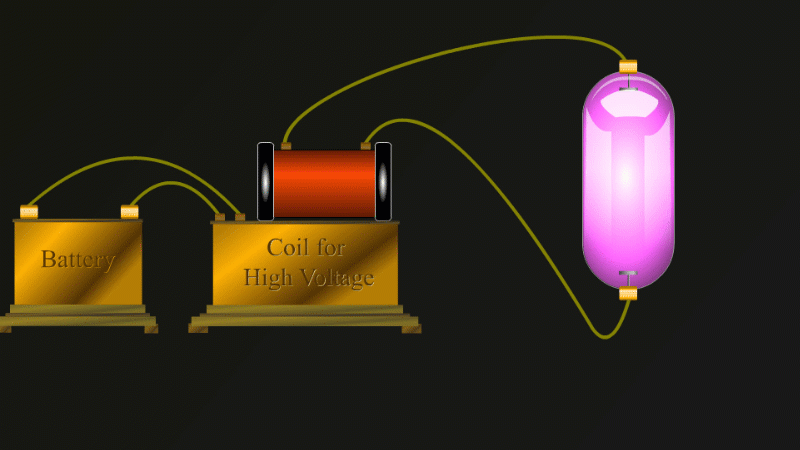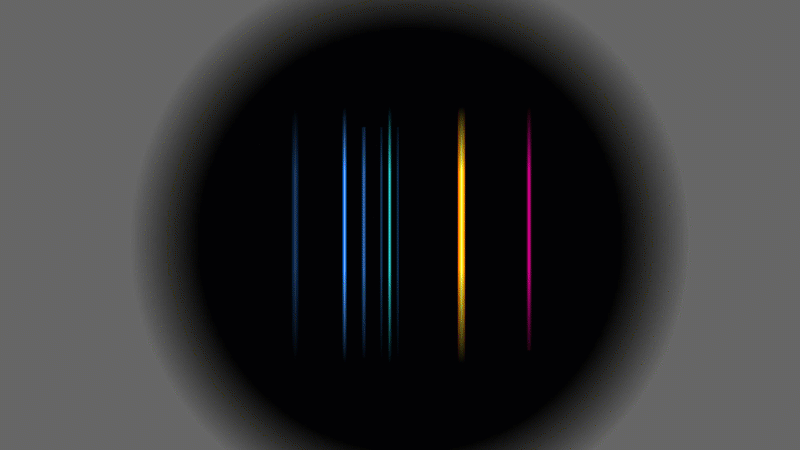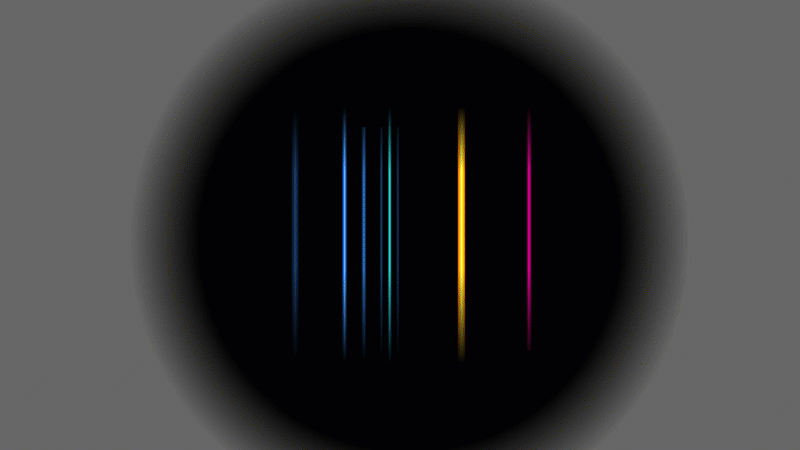
Several scientists found evidence that alpha particles and helium were linked somehow, but this is the experiment that Rutherford did in 1909. He used a radioactive gas that gave off alpha particles. When the alpha particles hit the walls of the container they grabbed a couple of spare electrons and turned into ordinary helium gas.
Rutherford showed that it was helium by putting a very high voltage across it (a bit like a Crooke's tube) and then splitting up the light given off into a pattern. It was the same pattern that hot helium gas would have made.
There was a third type of radiation discovered by the French scientist Paul Villard at the same time as alpha and beta had been discovered by Rutherford. This was a kind of invisible light, not a particle. The third type was called gamma radiation to be consistent with Rutherford's naming system.
-
Rutherford used magnets as well as electrically charged plates to discover the charge and relative mass of the alphas and betas.
Key animations from the video for you to use in front of a class
Alpha particles becoming helium gas
Rutherford distinguishes alpha and beta
Alpha and beta deflection
Proving the gas was helium
Radioactivity came before the nucleus
Rutherford’s early ideas about radiation
3.4 The sparkler analogy
Short video summary (2:31)
-
A radioactive substance is like a sparkler
The sparks are like the radiation
Radiation doesn’t go very far, doesn’t last very long, and doesn’t make other things radioactive
-
It's very common to get confused between the stuff that gives off the radiation and the radiation itself.
A sparkler's a good analogy. The sparkler itself is like the radioactive stuff - the sparks are like the radiation. The Radioactive stuff or "source" has a form - it can be a solid like a rock, or dust or a liquid or a gas.
Since it's stuff - it lasts - it sticks around for a long time. And it can move from place to place - you could carry it somewhere, or dust or gas might be blown by the wind.
As the sparkler gives off sparks, it runs out of its ability to sparkle, but it doesn't disappear. Though it does change. In the same way radioactive stuff doesn't disappear - it just runs out of its ability to emit radiation.
Alpha and beta particles are like the sparks. They go very fast but only last a tiny fraction of a second. They don't go very far. It's easy to block them and most importantly they don't make other things sparkly. In the same way radiation doesn't make the things it hits radioactive
Just like radiation, the sparks are pretty harmless unless you let a lot of them hit you.
Gamma radiation is a bit like the light from the sparkler. It gets dimmer the further away it is. And it gets dimmer the more stuff you put in the way.
Because the source and the radiation are completely different, you shouldn't talk about a cloud of radiation when you mean a cloud of radioactive dust or gas.
The precautions that you take are different, too. The source is only hazardous because of the radiation it gives off. So you want to stop it moving about and ending up somewhere it doesn't belong. This normally means keeping a source in a container, and if you're going to dispose of it, making sure it's in the form of a solid that can't dissolve in water.
To reduce the hazard from the radiation you want to reduce how much hits you. You can normally do this pretty easily by keeping your distance, putting something in the way (called shielding) and reducing your exposure time.
We'll find out more about dangers and risks in lesson 7.
-
The sparkler analogy is used several times throughout these lessons.
It’s introduced here because it’s so important to establish early on the difference between the properties of radioactive stuff and the radiation that stuff gives off.
Key animations from the video for you to use in front of a class
Gamma is like the light
The sparkler
The analogy with radioactivity
Differing precautions
3.5 Nuclear changes with alpha, beta, gamma
Short video summary (2:52)
-
A nucleus will decay if it is unstable
Decay means the nucleus changes (but doesn’t disappear), and gives off a particle at the same instant
An alpha particle is identical to a helium nucleus
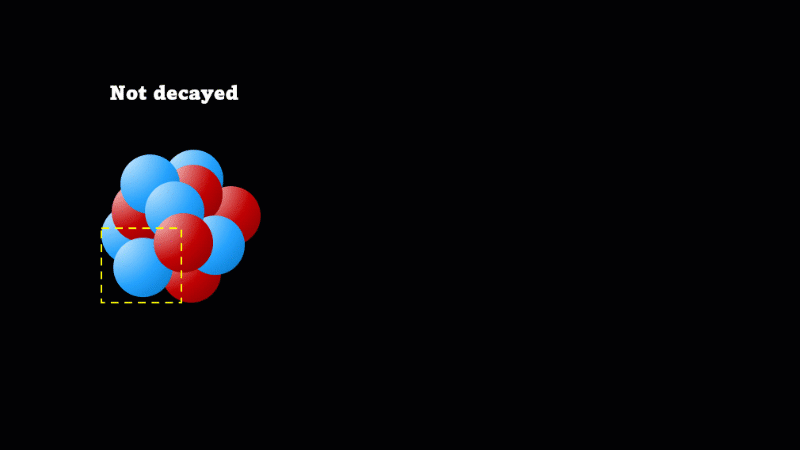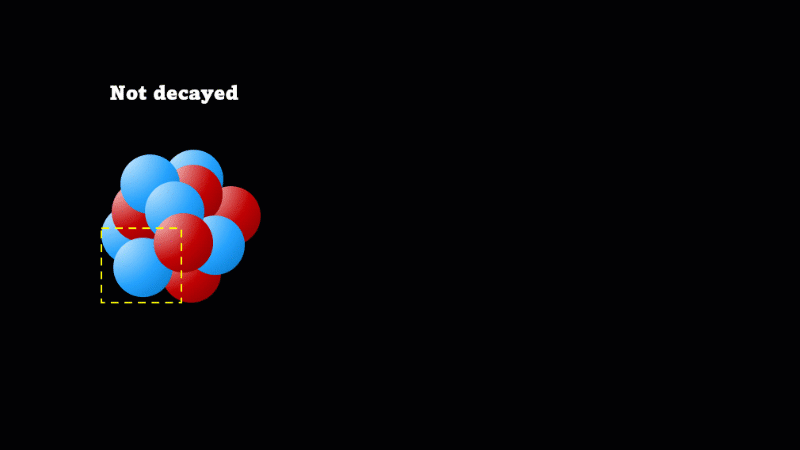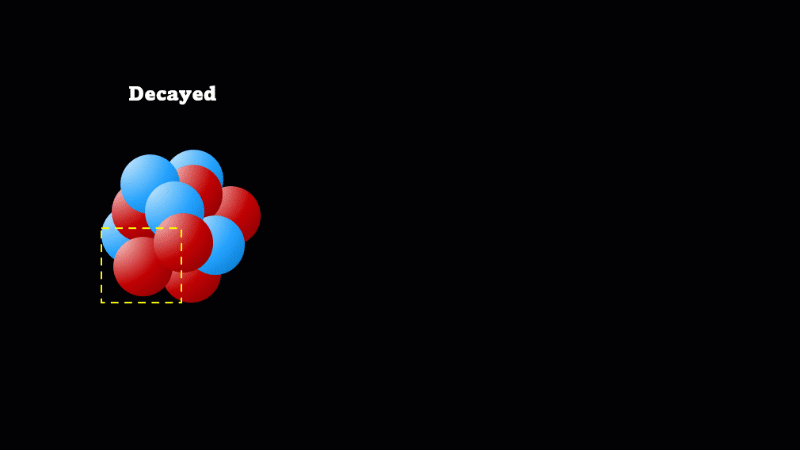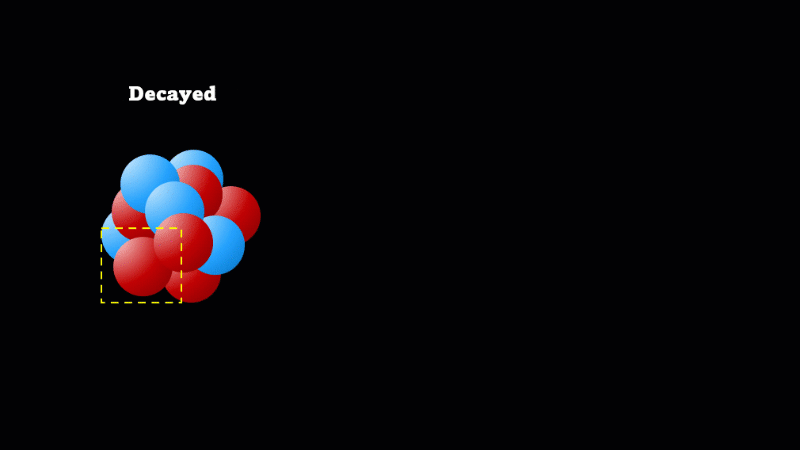
Alpha decay makes a big nucleus smaller
A beta particle is an electron that is created when a neutron changes into a proton
Beta decay makes the mix of protons and neutrons more stable
Gamma is a form of invisible, high energy light
Gamma decay makes the arrangement of protons and neutrons more stable
-
All nuclear radiation comes from the nucleus of an atom. The rest of the atom is still there, we just focus on the nucleus. We've seen there are three types of radiation, which Rutherford named alpha, beta and gamma.
An alpha particle is identical to the nucleus of a helium atom, consisting of two protons and two neutrons.
A beta particle is just a very fast-moving electron that come from changes in the nucleus, not the outer electrons.
And a gamma ray is like a tiny flash of invisible, very high-energy light.
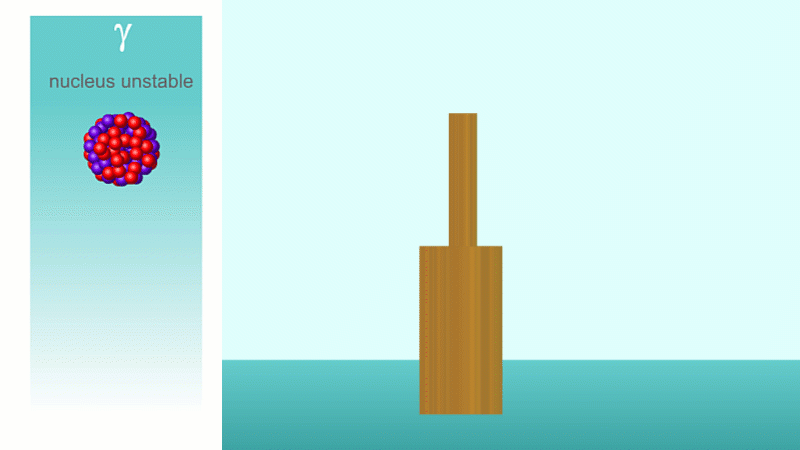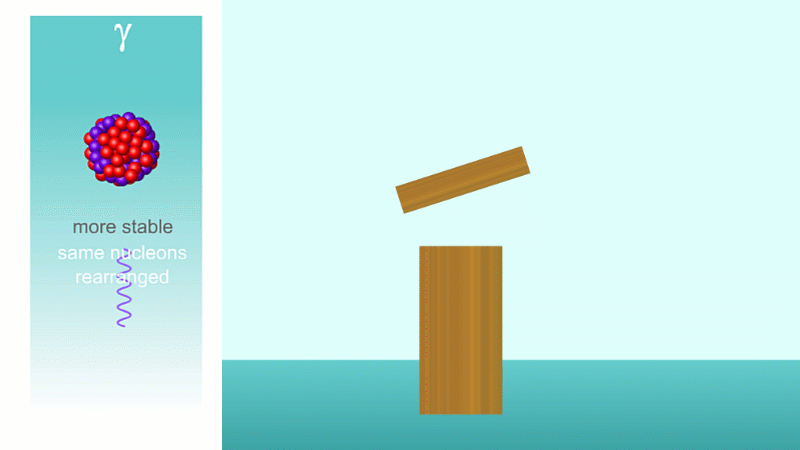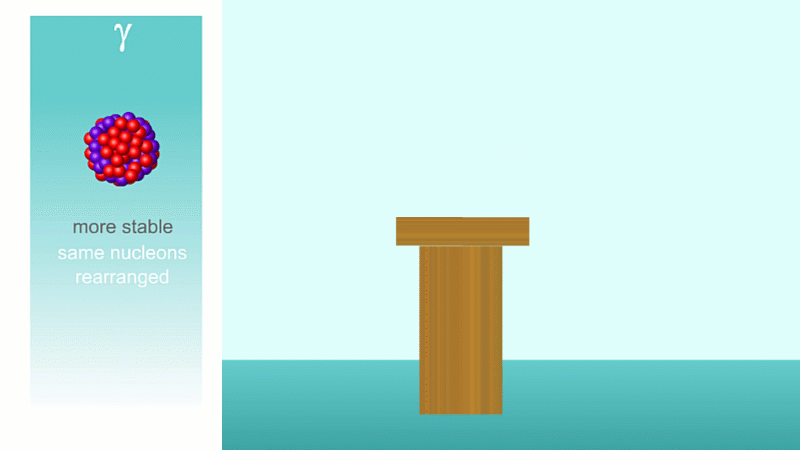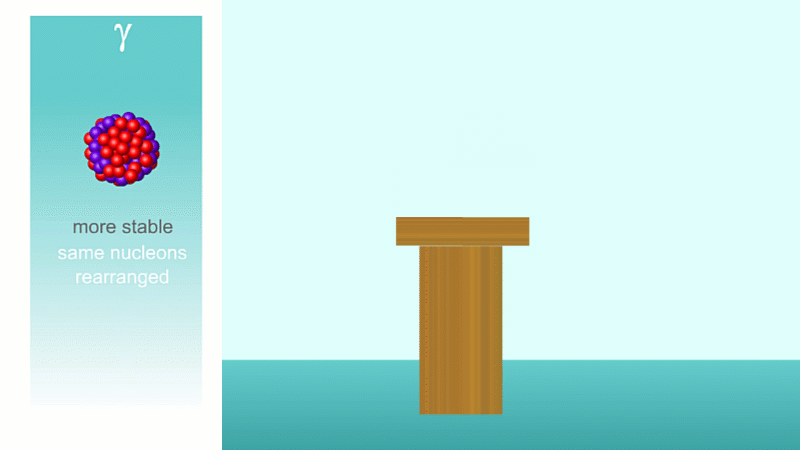
When a nucleus gives off a little 'spark' or a flash of radiation we say that the nucleus has 'decayed'. Decay doesn't mean that the nucleus (or the atom that its a part of) disappears - it just changes somehow. A nucleus decays because it's unstable.
If a nucleus is too big, it gives off an alpha particle. If the nucleus doesn’t have enough protons then a neutron may suddenly and spontaneously change into a proton. At exactly the same instant, an electron is created that's ejected from the nucleus at incredibly high speed. It's this high speed electron created in the nucleus that we detect as a beta particle.
Finally, after an alpha or beta particle has been given off, the arrangement of protons and neutrons (together known as nucleons because they make up the nucleus) may be unstable. The nucleus settles down into a lower energy, more stable arrangement and a gamma ray is created which takes off the energy that's been lost.
-
It's a very important idea that decay does not mean disappear. So even though the mass of undecayed nuclei will go down, that's at the expense of the mass of decayed nuclei going up. Alpha particles do take mass away, but unless you have a very pure sample of the isotope to start with, this is not easily measurable.
Only big nuclei decay by alpha emission. They often undergo beta decay immediately afterwards, which makes the ratio of protons to neutrons more stable.
Neutrons are very unstable in isolation, and decay into a proton with a half-life of about ten minutes. The protons in the nucleus stabilise the neutrons. If there aren't enough protons, at least one of the neutrons will turn into a proton and emit a beta particle.
Current scientific thinking suggests isolated protons are completely stable, but can be made less stable if they are surrounded by a small number of neutrons in a nucleus. This causes one of the protons to turn into a neutron and emit a beta plus particle, which is like an electron but with a positive charge.
Gamma decay can happen after alpha or beta decay. For a given alpha or beta decay, the proportion of subsequent gamma decays is not fixed.
Key animations from the video for you to use in front of a class
Alpha, beta, gamma summary
The meaning of nuclear decay
The structure of alpha, beta and gamma
Alpha decay and stability
Beta decay and stability
Gamma decay and stability
3.6 Penetration and ionisation
Short video summary (3:33)
-
The more ionising the radiation, the quicker it loses energy, and so the shorter the distance it travels
Alpha particles are highly ionising and so are stopped easily by 10 cm or so of air, or skin or paper
Beta particles are less ionising and so can make it through thin aluminium, but are stopped by thin lead
Gamma is much less ionising so can make it through thin lead, but is stopped by a few centimetres of it
-
Let's look at how alpha, beta and gamma radiation interact with whatever they hit.
An alpha particle is like a heavy concrete ball. It interacts very strongly with the ground it rolls over. It's got lots of energy, but it spends that energy in a short distance because it crushes the ground underneath it.
A beta particle is like a fast-moving golf ball. It interacts less strongly with the ground as it skips over it. It spends its energy over a long distance because it doesn't damage the ground very much.
Gamma radiation is a bit like a breeze. It hardly interacts with the ground at all. It can travel a long distance because it rarely loses any energy.
The way that alpha and beta particles lose energy is by ionising some of the atoms that they pass near. A typical alpha or beta particle will cause several thousand ionisations before it stops.
The ionisations cause “vapour trails” in a cloud chamber. Alpha particles make thick short vapour trails. The track gets thicker at the end as the alpha slows. This is because it's easier to grab an electron if you're not rushing past.
An alpha particle's double positive charge attracts electrons as it flies past. This is why alpha particles are highly ionising.
Beta particles typically move about a hundred times faster than alphas, so they're less good at causing ionisations. This means they make long, thin tracks of vapour in a cloud chamber.
All radiation will go through any material if it’s thin enough, and will be stopped by any material if it’s thick enough. But to compare penetrating powers we've traditionally looked at how alpha, beta and gamma fair when presented with air, paper, aluminium and lead.
As a rule of thumb, we say that alpha can go through a few centimetres of air but is stopped by paper. Beta will go through half a millimetre of aluminium but is stopped by the same thickness of lead. Gamma will go through thin lead, but can be stopped by a couple of centimetres of it.
Whereas alpha and beta are either stopped or not stopped, gamma tends to get dimmer as it goes through matter, a bit like light going through lots of sunglasses lenses.
It's a common misconception that gamma is incredibly penetrating and can only be stopped by lead. Any material can stop gamma if it's thick enough - it doesn't need to be strong or airtight. A few metres of straw will cut out 99.999% of gamma. It's just if we want our shielding to be thin then we want something dense like lead.
Sometimes gamma is less penerating than visible light. Light happily goes through 50 metres of water or tens of kilometers of air, but gamma can't. 8 metres of water or about 1 km of air is enough to almost completely cut out all but the highest-energy gamma radiation. That's why telescopes that look for gamma rays from space have to be in orbit - most gamma rays can't make it through the atmosphere.
-
Alpha particles are typically 5-100 times more energetic than beta particles, but because they are about 7000 times less massive they move at about 1/20 of a beta's speed.
This is still very fast - about 5% of the speed of light, compared with close to light speed for a typical beta.
A beta and alpha of the same energy would cause roughly the same total number of ionisations, the alpha would just do it in a shorter distance, which is why we say it's more ionising.
The mechanism for gamma ionisation is more subtle. A typical gamma will cause only a very small number of ionisations - typically one or two, but this means the ionised electron has a lot of energy, and it can go on and cause thousands of further ionisations. So most ionisations caused by gamma are actually secondary ones.
Key animations from the video for you to use in front of a class
Beta is less ionising
Alpha, beta, gamma with charge
Alpha is highly ionising
Gamma is very weakly ionising
Thickness matters more than material
Penetration through different materials
3.7 Detecting radiation
Short video summary (2:58)
-
Radiation can be detected by fogging film or using a Geiger counter
-
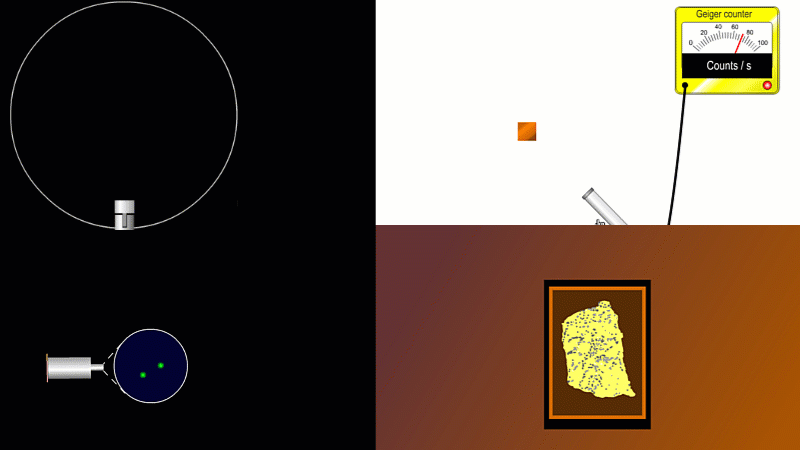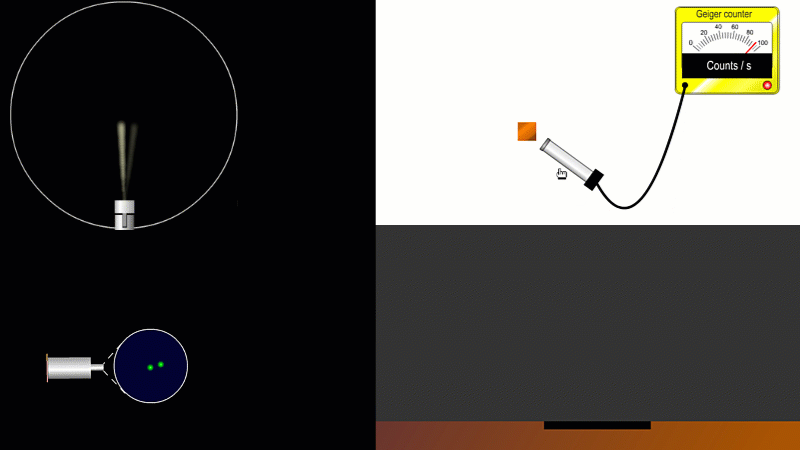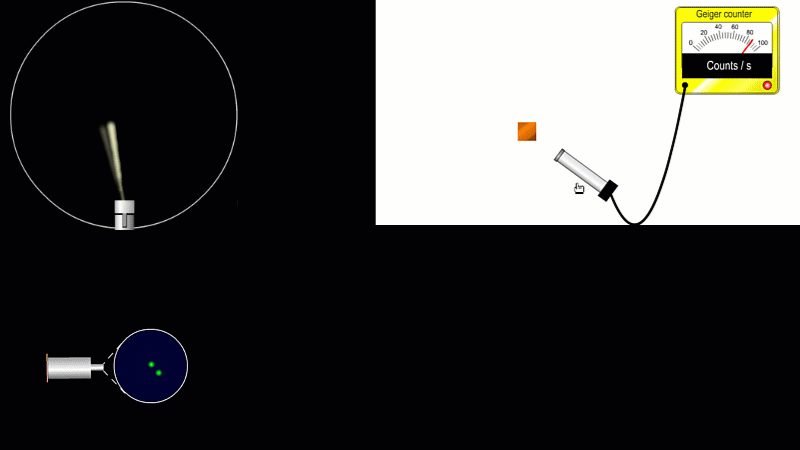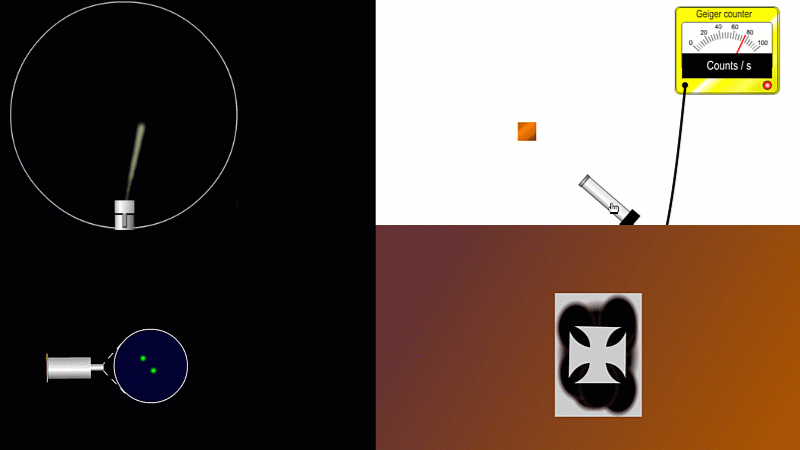
We've already come across several ways of detecting radiation. Henri Becquerel found out accidentally that radiation fogged film when he was doing another kind of experiment altogether. Ernest Marsden spent hours in a darkened room staring through a microscope at a fluorescent screen counting tiny flashes, and the actual paths that individual alpha or beta particles take can be detected by the trails of tiny droplets they leave behind as they shoot through a cloud chamber.
But the best known type of radiation detector is called a Geiger counter. The Geiger counter is made up of two parts: a tube which detects radiation and turns it into an electrical signal, and a counter which keeps track of the number of particles detected.
The detection part is called a Geiger-Muller tube, named after Rutherford's colleague Hans Geiger, who invented it in 1908, and his collaborator, Walter Muller, who helped him refine the design much later.
As you move the tube around a radioactive source, you can see it detects radiation in all directions. And as you move it closer and further away the reading goes up and down.
The G-M tube can only detect the small fraction of the radiation that actually hits it. The rest spreads out and is never counted. So you can use the G-M tube to find out how radiation changes with for example distance or shielding. But you can't use it to work out the total amount of radiation being given off.
The Geiger counter works by ionisation. The radiation enters through the end of the tube through a very thin screen which even lets alpha particles pass through it. Each alpha particle, say, causes ionisations of the low pressure gas inside the tube. A high voltage accelerates the electrons released, and causes a cascade of further ionisations. There are enough free electrons now for a small current to flow, and the detector registers a count.
G-M tubes are good at detecting individual alpha and beta particles, but they're not so good at detecting gamma radiation. This is because gamma rays produce very little ionisation in a gas.
To detect gamma it can help to turn the G-M on its side. A gamma ray can knock an electron off the metal wall of the G-M tube and cause an ion cascade and hence a count.
-
Students are no longer familiar with film, or the notion that it has to be developed. The advantage of film is that you don't need a high voltage, which is what most detection devices that rely on ionisation need.
The big disadvantage with film is that you have to wait for the film to be developed, which require specialist equipment. Film is rarely used nowadays for this reason.
Key animations from the video for you to use in front of a class
Electron avalanche in G-M tube
Four ways to detect radiation
Detecting gamma is difficult