Radioactivity Explained
4. Background radiation
Since the dawn of time
4.1 Sources of background radiation
4.2 The becquerel and the sievert
4.3 Compensating for background count in readings
4.1 Sources of background radiation
Short video summary (3:08)
-
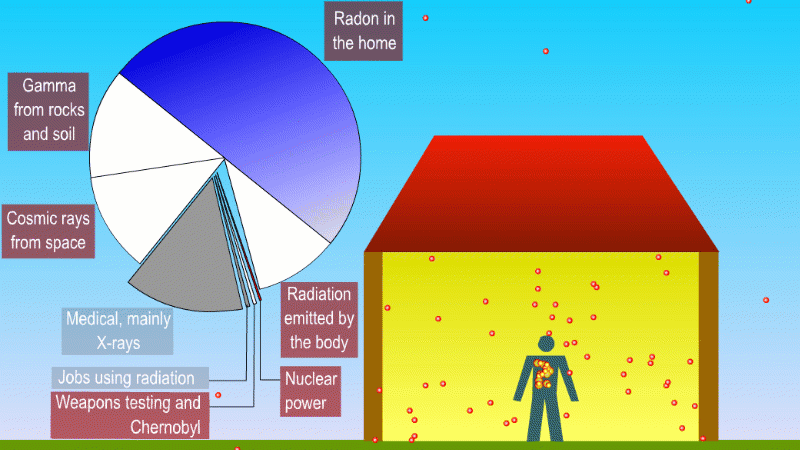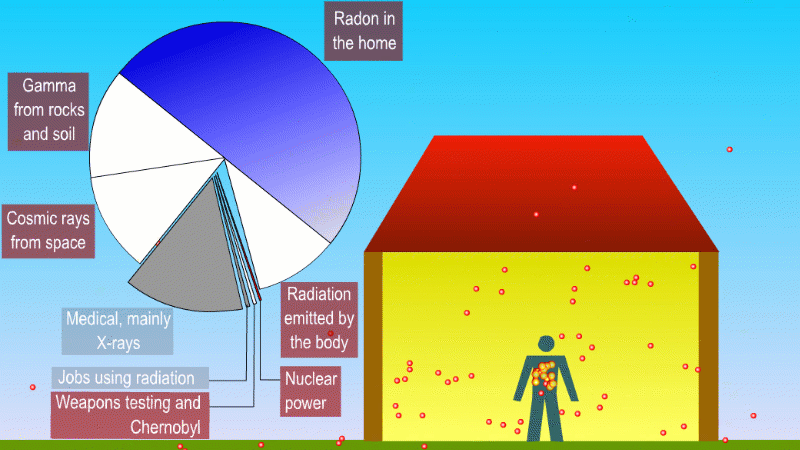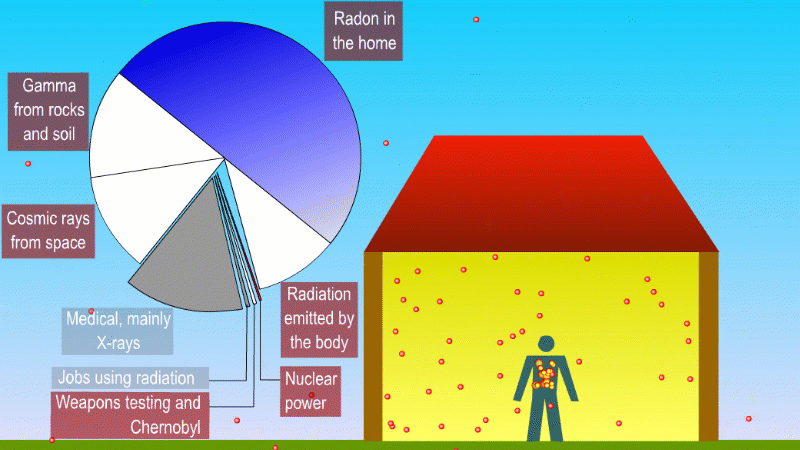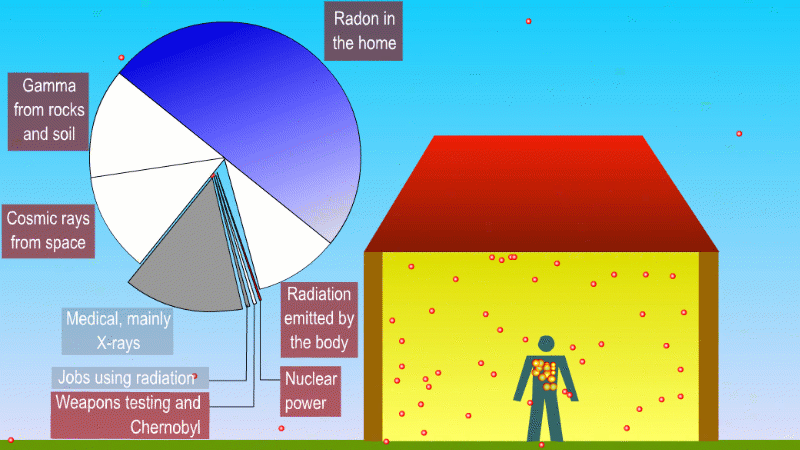
We receive a small continuous dose of background ionising radiation due to
- Alpha radiation from breathing in naturally occurring radon gas
- Gamma radiation from natural radioactive isotopes in rocks and soil
- Beta and gamma (mostly) from radioactive isotopes that naturally make up our bodies
- Cosmic rays
Medical procedures like X-rays and cancer treatment are averaged across the population
Nuclear power, nuclear weapons testing, and nuclear waste contribute a tiny amount to background dose
-
Every living thing is exposed to radiation all the time quite naturally. Background radiation is about the radiation dose a typical person would receive.
Alpha, beta and gamma all cause ionisation of atoms as they pass through them. As we'll see in lesson 7, unwanted ionisations in our cells can upset our cell chemistry and may cause health problems. So other forms of ionising radiation, for example X-rays, also get included when calculating background dose.
Radioactive isotopes have always been part of the Earth. The Sun and planets were formed about 4.5 billion years ago from the dust and gases of long-dead stars. The supernovas that ended these stars lives created the incredibly high temperatures and pressures needed for heavy nuclei to be fused from lighter particles like protons.
We've already met uranium, radium and polonium, but there are less exotic elements like potassium that have natural radioactive isotopes, as well as the stable ones we're used to.
Radioactive isotopes in the rocks and soil around us produce alpha, beta and gamma radiation, but the only form that can really hit people is gamma. An exception to this is naturally occuring radon, which is a gas and so we can breath it in. The alpha particles released by the radon gas can increases the risk of lung cancer. Different parts of the UK have different radon levels. There are regulations and precautions to control our exposure to radon.
Plants are mostly reorganised carbon dioxide with very small amounts of minerals from the soil they grow in. Carbon and some of the minerals have naturally radioactive isotopes. Animals eat plants, and we eat plants and animals, so we're made from very small quantities of radioactive isotopes, too.
One of the most important of these is potassium-40, which is a high energy beta and gamma emitter. We absorb about 3000 beta and gammas per second from potassium-40 in our body.
We also get bombarded with cosmic rays. Fast moving particles from deep space like protons create a shower of exotic particles when they collide with our atmosphere. These cosmic rays are a form of ionising radiation and so get added to our background dose. If you live at high altitude or take lots of flights you're exposed to more cosmic rays.
If you have a medical procedure like an X-ray this is also added to the background dose. But these are averaged across the population, rather than tracked for each person.
Other human activity like nuclear weapons testing, nuclear accidents and nuclear power plants add almost nothing to background radiation.
-
You can divide background radiation into the radiation from nearby radioactive stuff, and everything else.
As we mention in section 8.1 on nuclear equations, all the naturally occurring radioactive isotopes found on Earth either have a very long half-life - and so survive from the ancestral supernovae that produced them - or are radioactive decay products with those long-lived isotopes as the ultimate parent.
Radioactive isotopes like those of radon, radium and polonium were produced in these ancient supernovae, but had long since decayed to stable isotopes before the Earth was formed. The instances of these isotopes we find on Earth now are all produced as a result of the decay of very long-lived parent nuclei, like uranium-238.
Key animations from the video for you to use in front of a class
Cosmic ray formation
Sources of background radiation
Cosmic rays in pie chart
Gamma from rocks and soil
Radiation emitted by the body
Radon in the home
4.2 The becquerel and the sievert
Short video summary (1:32)
-
Radioactivity is a measure of how much radiation a radioactive substance gives off
Radioactivity is measured in becquerels (Bq). 1 Bq is one count per second
Radiation dose is a measure of the potential harm being exposed to radiation may cause
Radiation dose is measured in sieverts (Sv). It depends on the quantity and type of radiation
-
There are two concepts that we need to know about when measuring radiation. Radioactivity is all about things - how much radiation a radioactive source gives off. Radiation dose is all about people - how much radiation hits someone.
The unit of radioactivity is the becquerel (Bq). The unit of radiation dose is the sievert (Sv).
The becquerel is quite a simple unit - it's just the counts per second, and it's what a Geiger counter measures. If the Geiger counter detects 80 particles per second then we say that's 80 Bq.
We just need to be a little bit careful that a Geiger counter can't measure total radioactivity because most of the radiation doesn't actually hit it, and so can't be counted.
Radiation dose is quite involved, but you don't need to understand it in much detail. It's a measure of the potential harm a dose of radiation can do, and it depends on things like the type of radiation (alpha, beta or gamma), its energy, how much of it hits you and in which part of your body.
One sievert is a very large dose, so most doses are given in millisieverts (mSv)
The average background radiation dose each year is about 0.002 sieverts or 2 millisieverts.
-
The becquerel is used to measure the number of alphas, betas or gammas (or other more exotic radiation) given off per second. If they hit a detector then each one is referred to as a "count".
Even though the becquerel is defined in terms of the number of counts per second, it can also be used as a unit for the total number of "counts" under some circumstances. The becquerel is the unit for all these quantities:
- the count rate detected by a Geiger counter from a source, even if most of the radiation doesn't hit the counter
- the total number of alphas etc. given off by a source per second - this is what's meant by "the radioactivity" of the source
- the total amount of alphas, etc over a period of time from many different sources, for example the total amount of radiation given off by all atomic tests
The becquerel is agnostic about where the radiation ends up, but the sievert is only interested in the radiation that actually hits an individual - that's what we mean by dose.
There are actually two units of radiation dose - the gray (Gy) and the sievert (Sv). They both have units of J/kg - in other words they are a measure of the energy absorbed by a person per kg of the tissues that have done the absorption.
The gray is only about energy, and doesn't care about harm. The sievert tries to put a number on risk from a radiation dose. The sievert can actually be used to measure three related quantities:
- Equivalent dose - energy per kilogram taking into account the type of radiation (alpha is assumed to be 20x as harmful as beta or gamma)
- Effective dose - equivalent dose adjusted for which organs or tissues are exposed
- Committed dose - total dose over an extended period of time if the radioactive source is inside you
The sievert is most useful for comparing whole-body doses over extended periods of time (i.e. external gamma) rather than short bursts of radiation limited to a small part of the body.
For this reason the very intense but very local doses people receive for cancer treatment are measured in grays not sieverts.
Key animations from the video for you to use in front of a class
Radioactivity and dose are different
4.3 Compensating for background count in readings
Short video summary (1:08)
-
Background count changes with location and time
You should subtract the background count from an experimental count rate if it will make a difference
-
If you leave a Geiger counter on it will gradually tick up counts due to background radiation.
If you left it counting for say 10 minutes then you could calculate the background count rate - in other words the number of counts per second.
The background count rate in becquerels is the number of counts divided by the time in seconds.
There are 600 seconds in 10 minutes.
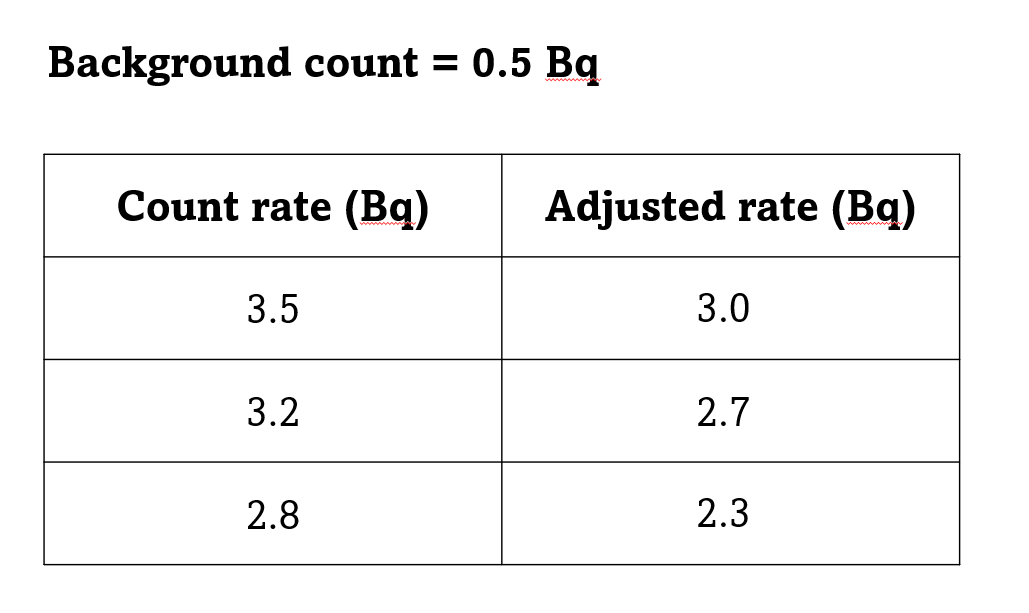
If the total number of counts in 10 minutes was 317, then the count rate would be 317/600 = 0.5 Bq.
So really you should reduce any readings you take by 0.5 becquerels to compensate for the background radiation. This is only really worthwhile if the count rate for your measurements is quite low.
The measured background count will change with time, location and the particular Geiger counter you're using. So really you should take a background reading every time, and not just assume that you know it.
-
Ideally you want to keep the detector in one place and then bring the radioactive source up to it. This makes sure that you're compensating for the correct background count.
Key animations from the video for you to use in front of a class
Geiger counter ticking up randomly
Adjusting for background count