Radioactivity Explained
6. Uses of nuclear radiation
Nothing else will do
6.1 Non-medical uses
6.2 Medical uses
6.1 Non-medical uses
Short video summary (1:50)
-
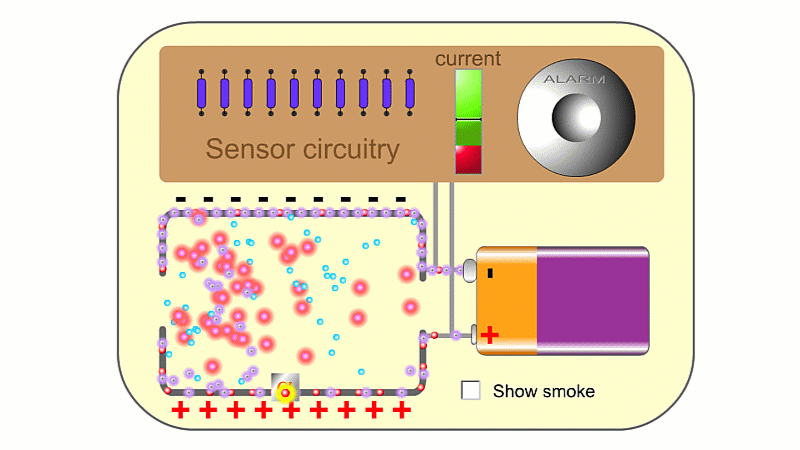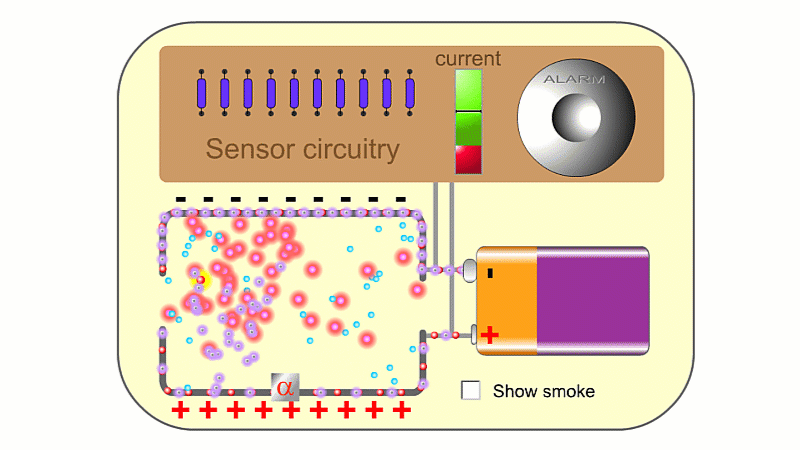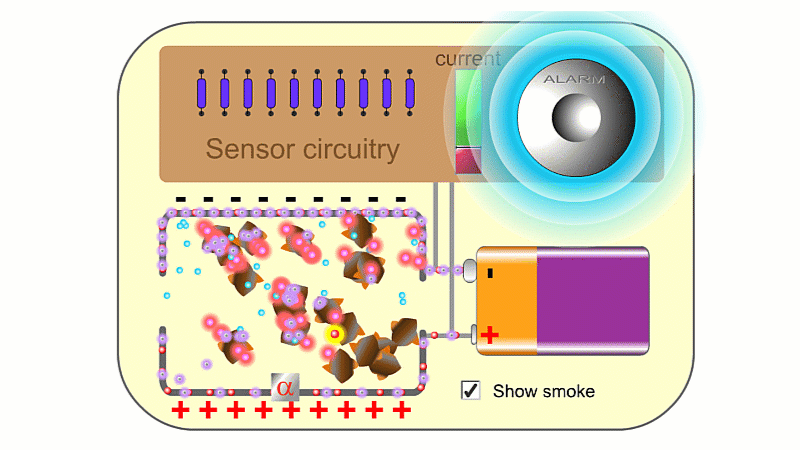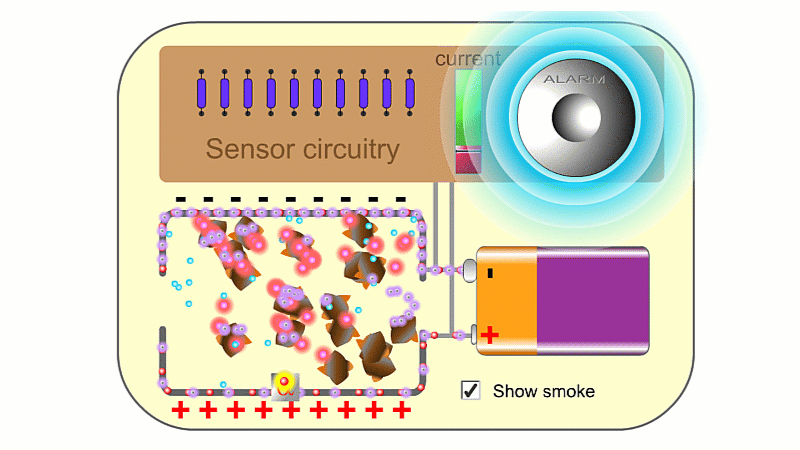
Alpha is used when you want lots of ionisation - for example a smoke detector
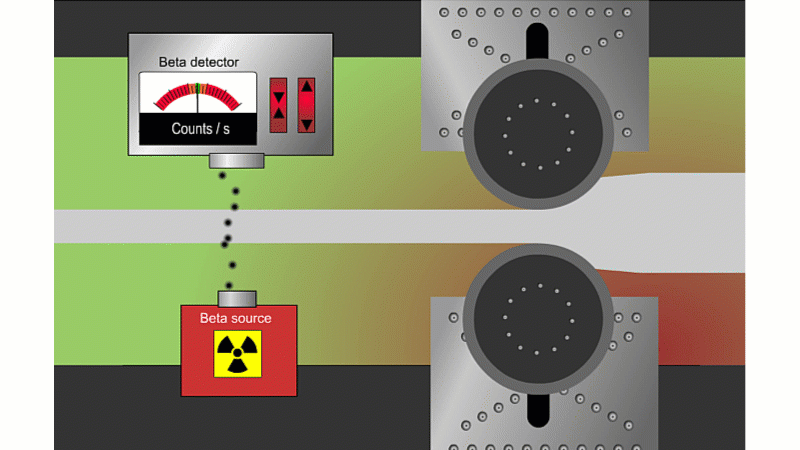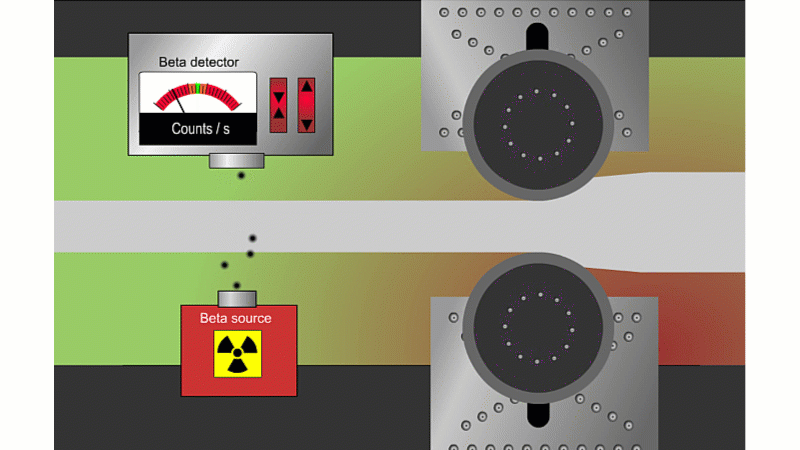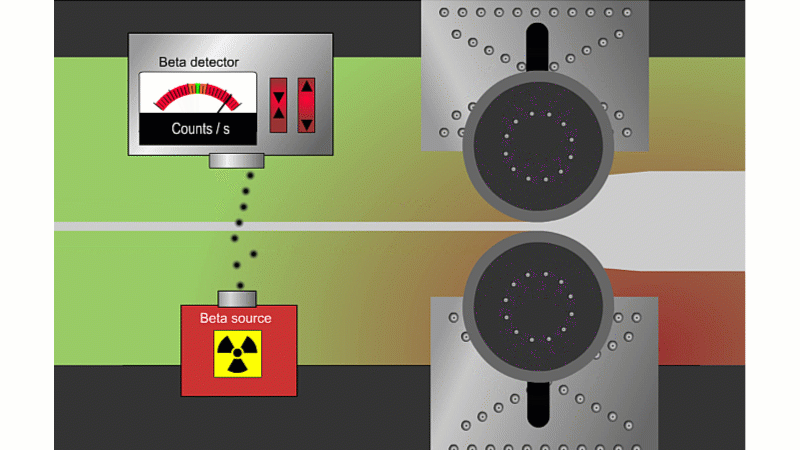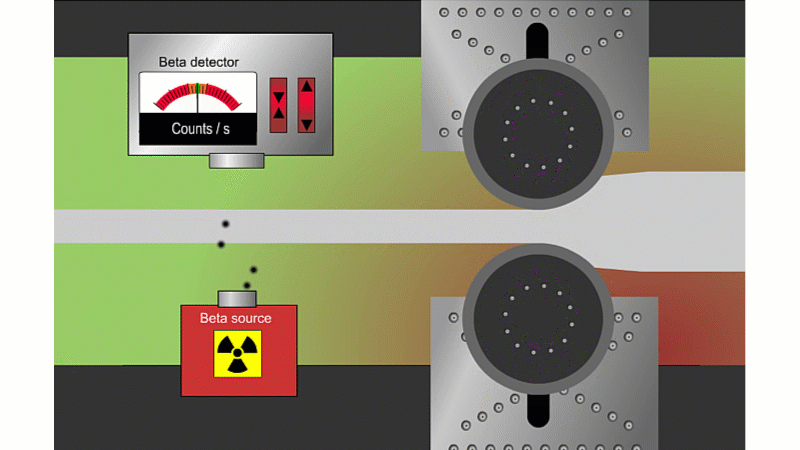
Beta can pass through thin sheets of paper or metal, so it's good for measuring thickness
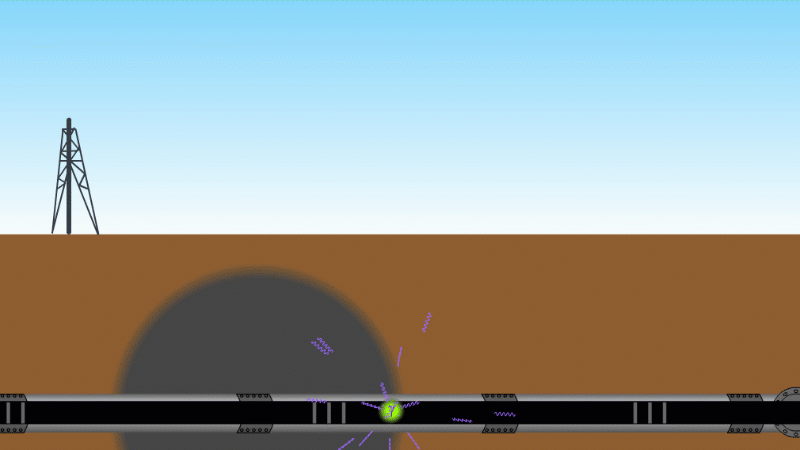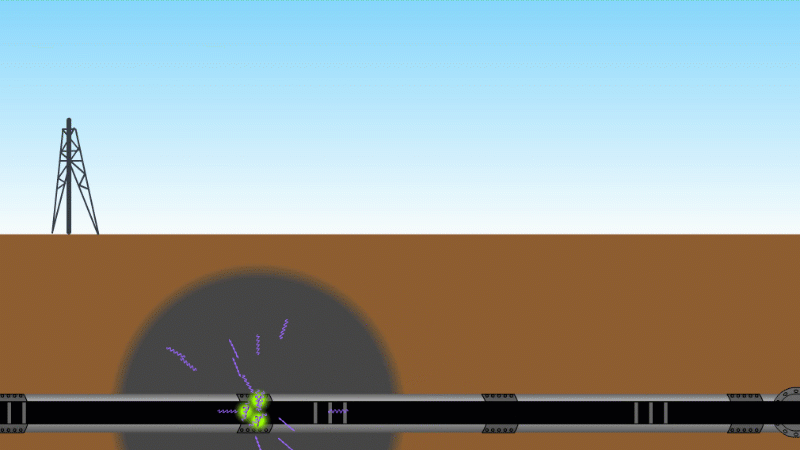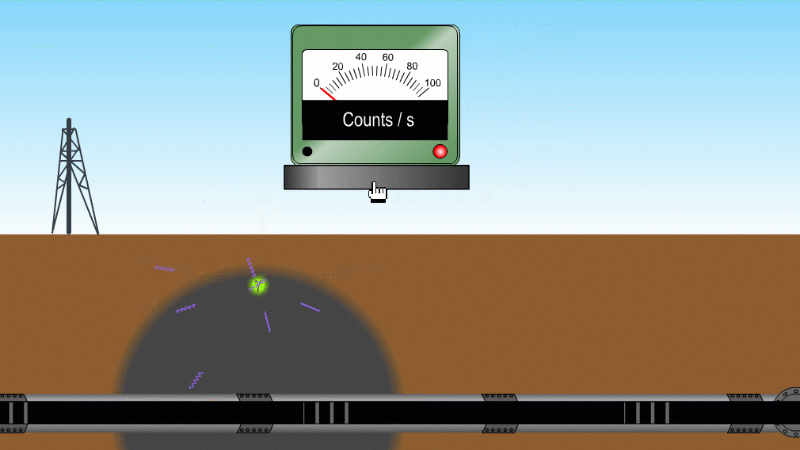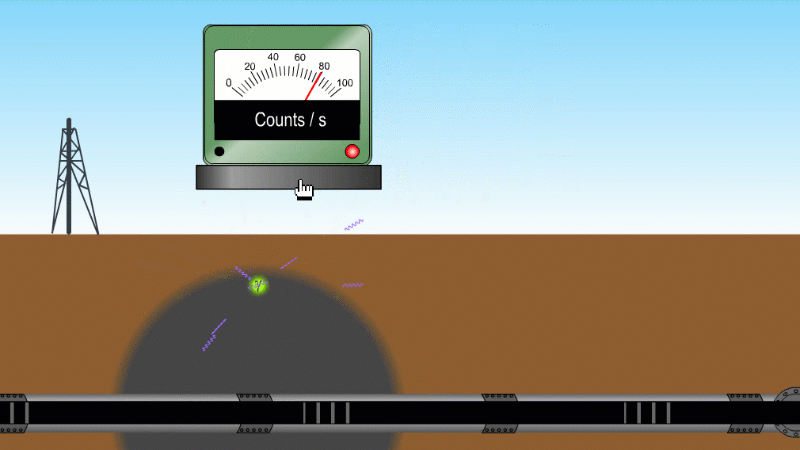
Gamma can pass through a metre or two of rock, soil or water, so it's good as a tracer
Gamma can also penetrate delicate equipment and kill bacteria without causing too much heating
-
Alpha, beta and gamma radiation have individual properties that are each good for certain jobs.
Alpha particles are very ionising. In a smoke detector they can create so many ions that the air conducts slightly and a battery can create a very small current. If smoke enters, then electrically charged air ions are attracted to the much larger smoke particles, and stick to them. This means the air can't conduct as well, clever circuitry detects the drop in current and sets the smoke alarm off.
Beta particles can go through thin metal, but how much depends on the thickness. Alpha couldn't go through at all, and gamma wouldn't change enough with thickness. A beta thickness gauge fires beta particles through thin metal or paper as it shoots by.
If the metal is a little thicker than it should be, then the beta reading drops and the machine automatically adjusts to make it thinner. If it's too thin then the beta reading increases and the machine automatically adjusts to make it thicker. The advantage is that you don't need to stop the rolling process to check the metal is the right thickness.
Gamma can pass through things quite easily and be measured. For example by releasing a gamma source into an oil pipeline you can locate a leak, even if it isn't visible at the surface. Because gamma can kill bacteria with very little heating you can use it to sterilise food and medical equipment without damaging them. Remember our sparkler analogy - no radioactive material touches the food or the medical equipment - and radiation doesn't make other things radioactive.
-
You sometimes see teaching resources and even exam questions incorrectly imply that smoke alarms work because the smoke blocks the alpha particles themselves, rather than mopping up ions.
You need a high voltage (~500 V) to detect radiation, and smoke alarms typically run off 9 V. So smoke alarms can't detect radiation directly. The alpha source creates so many ions that it makes the air conduct slightly, and so a 9 V battery can make a small current pass continuously, which is detected. If smoke particles mop up the ions then the air stops conducting, the current drops and the alarm goes off.
Key animations from the video for you to use in front of a class
Gamma tracer
Alpha used in smoke detector
Beta thickness gauge
Gamma sterilisation
6.2 Medical uses
Short video summary (3:00)
-
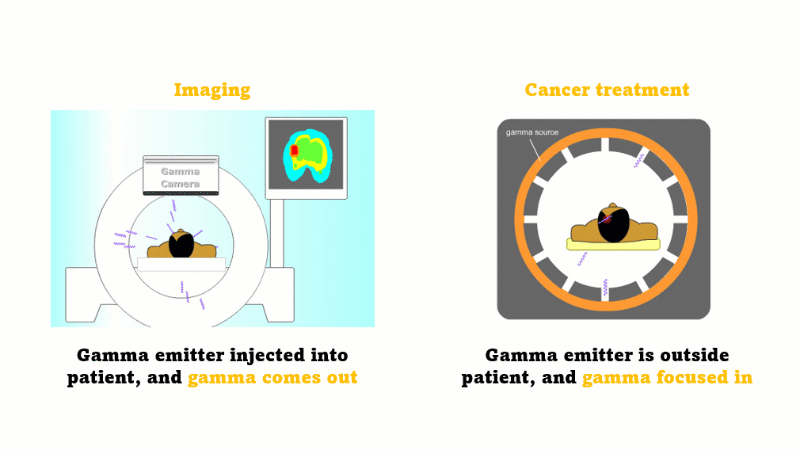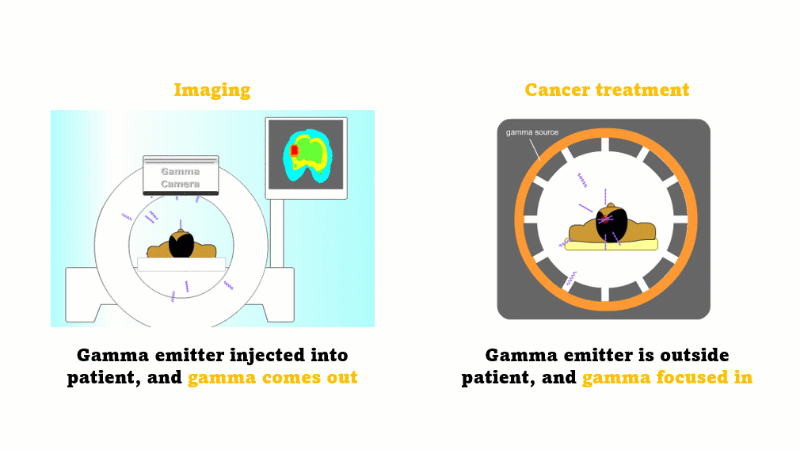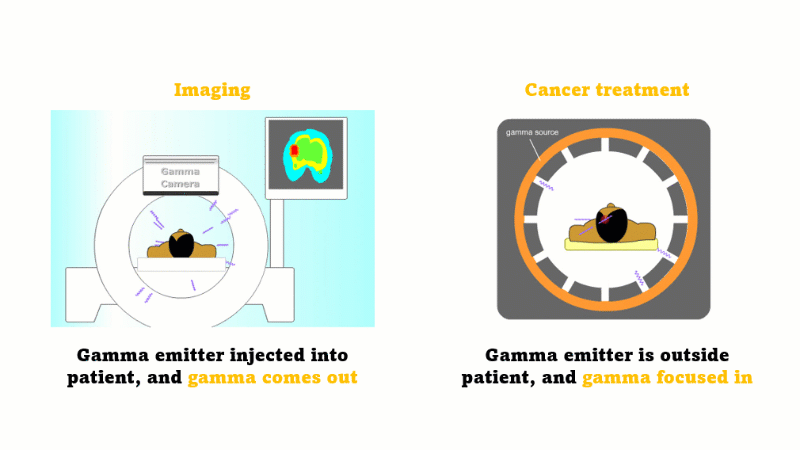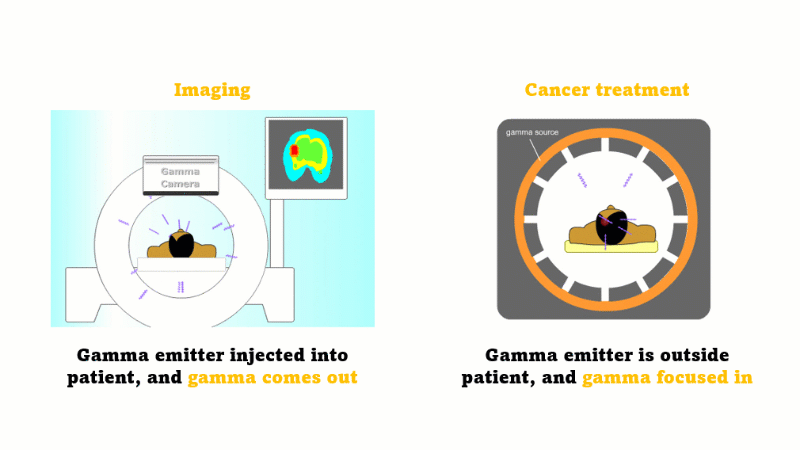
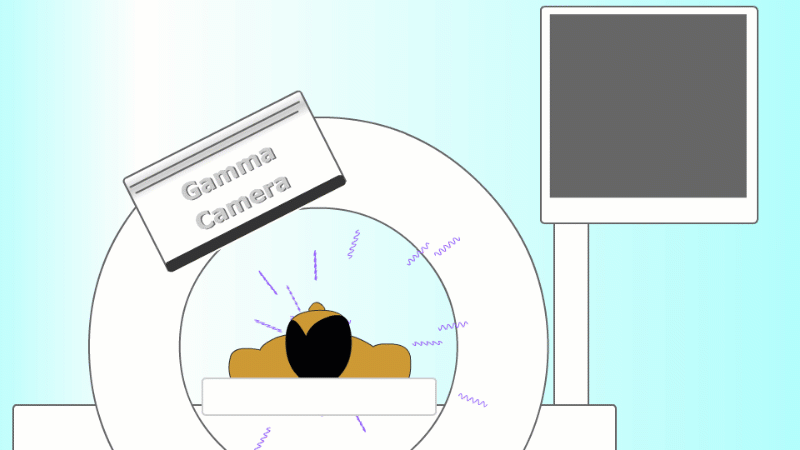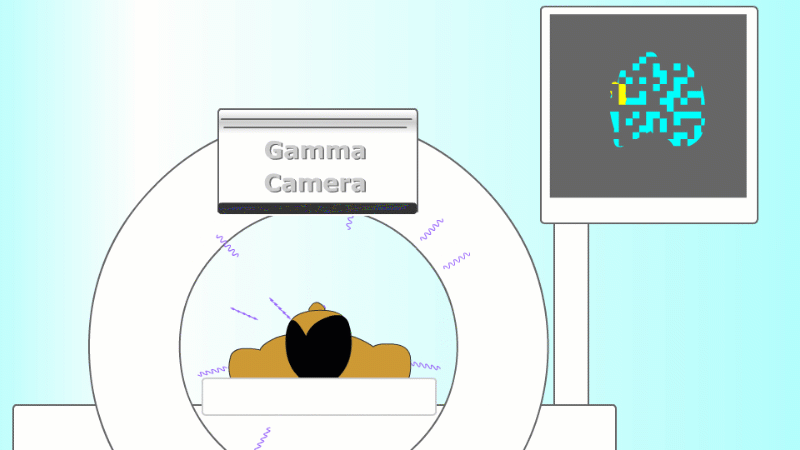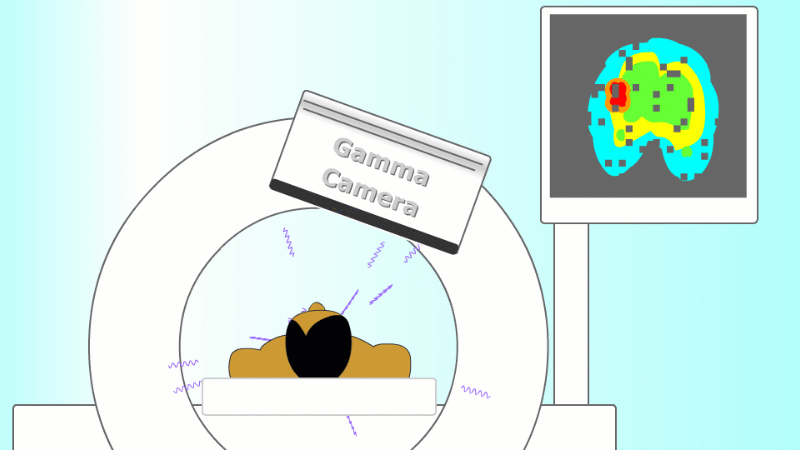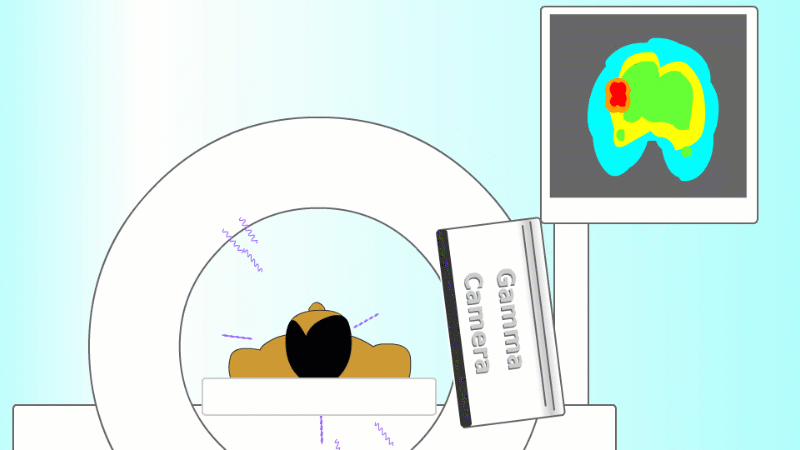
Gamma radiation is used for medical imaging
A gamma emitter is injected into the patient, and the gamma rays that leave the patient's body are captured using a special gamma camera
Sometimes the gamma is produced indirectly when beta plus particles and electrons annihilate each other, which is how a PET scan works
Gamma is also used to treat cancer
A gamma source outside the patient is focused inwards towards the cancer and kills the cancer cells
Beta emitters can also be used to treat cancer if they are injected very close to the cancer site
-
In medicine radiation is used for imaging and treating cancer.
For imaging, a gamma emitter is injected into the patient. The gamma rays come out and are captured using a gamma camera.
For treating cancer the gamma emitter is outside the patient. The gamma rays are focused inward towards the cancer. The gammas are most intense at the site of the cancer but the surrounding tissues receive a far smaller dose.
Only gamma radiation can penetrate deep into the body. But sometimes a beam of beta radiation can be used for surface cancers, or little capsules of beta emitters can be injected around the site of a cancer. The cancer gets a bigger dose than the surrounding tissues.
Let's look at imaging in a bit more detail. We always use an isotope that causes gamma rays to be emitted. Sometimes that's an isotope that emits gamma directly, and sometimes the isotope causes gamma rays to be produced indirectly.
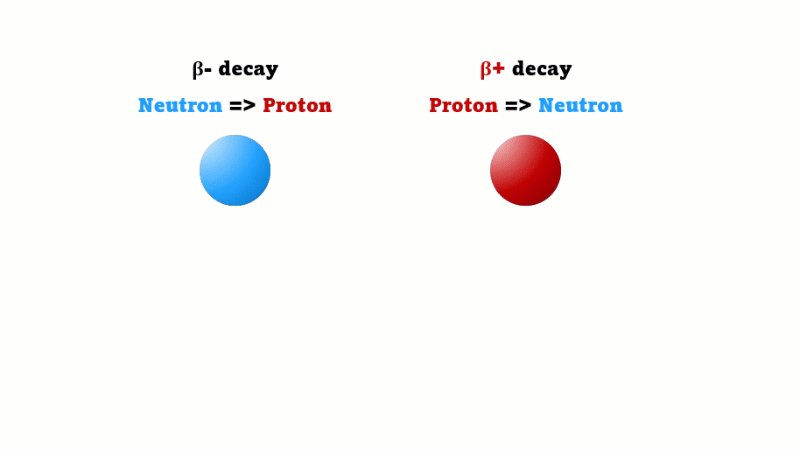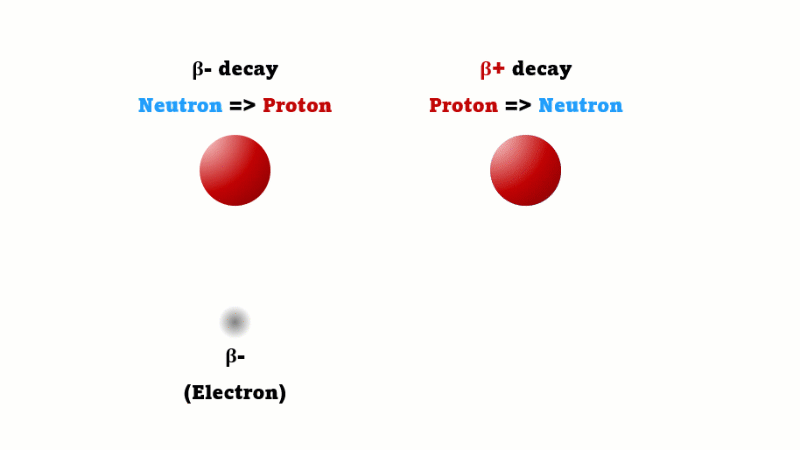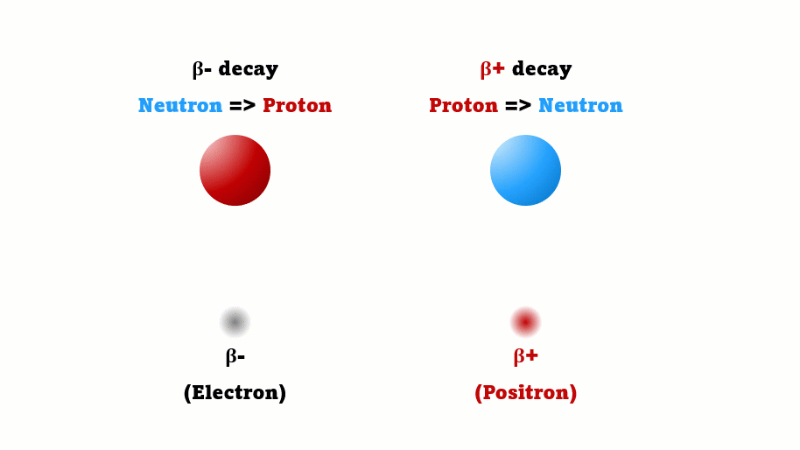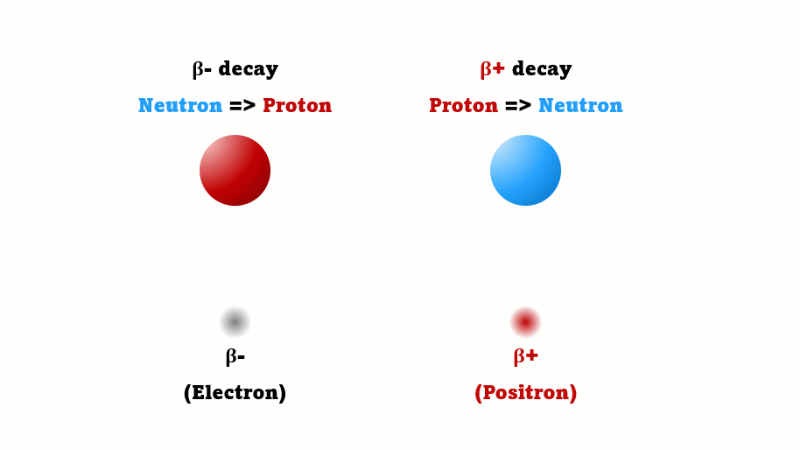
This is what happens with a PET scan. PET stands for positron emission tomography. A positron is a special kind of electron that doesn't exist on Earth naturally. It's exactly the same as an electron except it has a positive charge.
A positron is also called a beta-plus particle - they're just two names for the same thing. A regular beta (beta minus) is created when one neutron in a nucleus changes into a proton. A beta plus is created in a mirror image process - when one proton in a nucleus changes into a neutron.
We've seen in lesson 5 that beta-plus emitters have to be made artificially using a sophisticated machine called a cyclotron. And that, because beta plus emitters have a very short half-life - typically under 2 hours - the cyclotron needs to be close to or in the hospital.
Every positron that's given off immediately comes across a regular electron and the two annihilate each other producing two gamma rays. It's these gamma rays that are detected.
The beta-plus emitting isotope is chemically attached to something the body uses like sugar molecules and is injected into the patient . This 'tracer' tends to concentrate where the cancer is because cancers use a lot of energy to grow and they get this from sugar.
The gamma rays that are given off leave the body and are detected by a special piece of apparatus called a gamma camera. As more and more gamma rays are captured an image is built up of the cancer site. PET scanners are set up to record each pair of gammas on opposite sides of the patient to help locate the exact point they came from.
-
It's interesting that radiation is both a health risk and a treatment. The reason is the same - radiation can disrupt cell chemistry. In small quantities this can damage DNA without killing the cell in such a way that very, very, very rarely the cell can divide unchecked as a tumour.
Cells that are naturally prone to divide, like cancer cells, are most susceptable to being damaged by radiation. This is why radiation therapy is somewhat selective about the cells it attacks. It's also why it makes your hair fall out and feel very ill, because hair follicles and the lining of your gut are also places where cells tend to be dividing.
Gamma for therapy is more often produced by a particle accelerator, rather than by a radioisotope. The advantages are similar to those of an electromagnet, in that you can turn it off and adjust its intensity.
Key animations from the video for you to use in front of a class
Beta minus and beta plus
Comparing imaging and treatment
Beta cancer therapy with beads
Beta plus annihilation producing gamma
Injecting a medical tracer
Imaging with a gamma camera