Radioactivity Explained
7. Dangers and risks
Safe but scary
7.1 Contamination and irradiation
7.2 Effects of radiation on living things
7.3 Managing risks
7.4 Risk perception
7.1 Contamination and irradiation
Short video summary (3:24)
-
Contamination is about getting radioactive material where it shouldn't be
Contamination can be prevented by making sure the material is a solid that can't dissolve in water, and then keeping track of it
You can remove contamination by washing the radioactive material off
Irradiation is about the radiation itself hitting someone or something
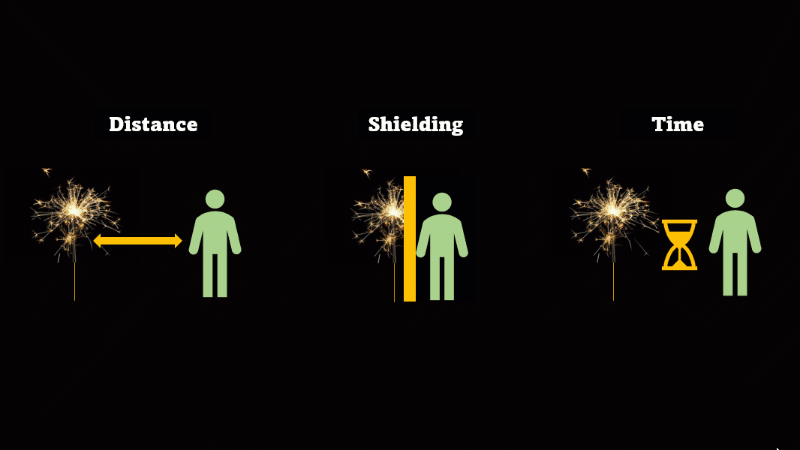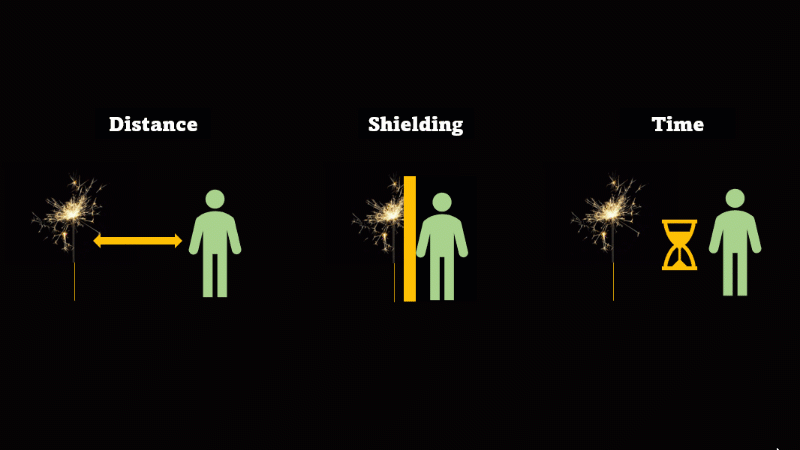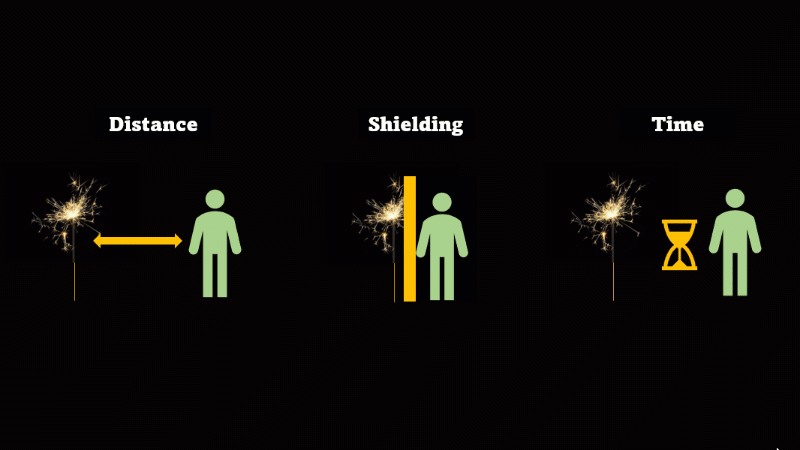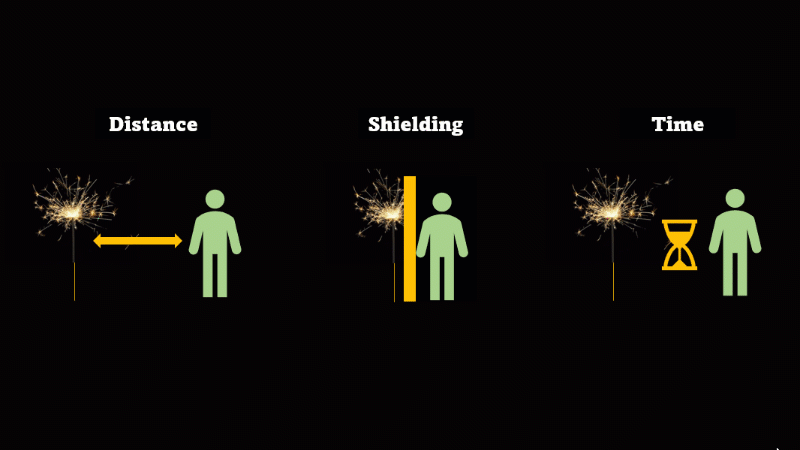
If you have to be near radioactive material then you reduce irradiation by using shielding, increasing distance, and reducing exposure time
-
Remember our sparkler analogy from lesson 3. There are two parts: The sparkler represents the radioactive stuff - and the sparks represent the radiation itself.
If radioactive stuff ends up in or on something where it shouldn't be we say that thing has been contaminated. If radiation hits something we say that thing has been irradiated. Radioactive stuff and radiation are completely different.
Radioactive stuff has a form like a dust or liquid or more rarely a gas. Because it's stuff it sticks around, so it can move long distances over hours, days or months. It will eventually stop being radioactive but it never disappears.
Radiation lasts a tiny fraction of a second. It doesn't go very far - even gamma - and it's easy to block.
It doesn't make other things radioactive - even if huge amounts of radiation have hit something. And it's pretty harmless in small doses - remember we have 5 - 10 thousand radioactive decays per second going on in our bodies for our whole lives just due to the radioactive isotopes that are naturally part of us.
If we want to avoid irradiation from an external source then there are some simple things we can do:
First, just keep away from the source. Alpha can't go more than a few centimetres through air, beta only a metre or so, and gamma spreads out like light as the square of the distance, so it's a hundred times dimmer at 10 metres than it is at 1 metre.
If you have to be near a source - then just put something in the way - this is called shielding.
And if you can't keep away and you can't use shielding then reduce the amount of time you spend being irradiated.
Contamination is all about having radioactive stuff where it shouldn't be. Water, food and dust are already naturally radioactive but if we add some more radioactive materials that shouldn't be there then we say we these things have been contaminated. So if we drink the water, eat the food or get dust on us then we get irradiated.
This is a problem because we can't escape from being irradiated if the radiation source moves around with us - we can't use distance or shielding or time to reduce our dose.
The best way to stop contamination is to make sure radioactive materials are solids that can't dissolve in water - this keeps them in one place and you can keep track of them.
If something gets contaminated with radioactive dust, say, then you can wash it off and you stop being irradiated by it. You can let the radioactive dust mix in with the millions of tonnes of naturally radioactive material that's part of the environment or you can collect it and put it somewhere away from people.
-
Common fears about radioactivity come from assuming that radiation itself has the same properties as radioactive stuff. People think that radiation can hang around like a gas, move long distances, and then 'infect' other things, making them radioactive.
You don't need contamination to get irradiated - you can simply be too close to an unshielded source - but the problem with contamination is that you can't escape the irradiation unless you remove the contaminant. Radiation is silent and invisible so without special detectors you often can't tell that something has been contaminated with, for example, radioactive dust.
Key animations from the video for you to use in front of a class
Examples of contamination
Revising the sparkler analogy
Avoiding irradiation
Contaminated and irradiated
Stopping contamination
7.2 Effects of radiation on living things
Short video summary (3:06)
-
Nuclear radiation is hazardous because of the ionisation it causes inside cells
The ionisation disrupts chemical reactions that make cells work
In high doses this can cause radiation sickness
In low doses it can damage DNA, which can increase the risk of cancer
Alpha and beta radiation are more hazardous if the radioactive material is inside the body
Gamma can be hazardous both inside and outside the body
-
Radiation can be hazardous to health. It's the ionisation that's the problem. Ionisation frees up electrons to interfere with the chemistry that makes our cells work. In high doses - above about 500 mSv or 0.5 Sv - this can stop our organs functioning and make us very ill - sometimes fatally.
In 1986 a reactor at the Chernobyl nuclear power plant in Ukraine caught fire after inexperienced operators late at night tried to make the reactor do things they had been explicitly told to avoid. Firefighters were lowered in helicopters over the burning reactor core and they were irradiated with up to 10,000 mSv - that's 10 whole sieverts of gamma and neutron radiation. 28 out of 134 of them died of acute radiation syndrome within weeks of their exposure, but most made a full recovery and went on to have healthy children.
In a nuclear reactor, radioactive iodine is produced which can accumulate in the thyroid gland if you eat contaminated food. 15 people, who were children at the time of the accident, died of thyroid cancer over the following 25 years, and there are predicted to be a total of 160 excess deaths, equivalent to about 3 a year. There has been no proven increase in any other cancer, including in the firefighters.
If you're asked about health effects of radiation in an exam then this is the kind of thing you should put to get the marks:
"Radiation damages DNA which can lead to genetic mutations that cause cancer"
A much more accurate but less snappy response would be:
"Radiation sometimes damages DNA without killing the cell, which very, very rarely can lead to a particular type of genetic mutation that causes cancer years later"
Every cell in our body experiences about 10 million instances of DNA damage each year due to natural chemical processes. For background radiation the equivalent figure is about 1 piece of DNA damage per year.
However, we still do what we can to minimise the risk. Alpha and beta are only really hazardous inside the body - alpha can't make it through skin and beta will only penetrate the first few millimetres. To avoid alpha and beta risks you want to reduce ingesting or breathing in contaminated water, food or air.
Gamma radiation is more hazardous outside the body - even though some of it will pass straight through you. To avoid the risk from gamma you use shielding, increased distance, and reduced exposure time to minimise irradiation.
-
By any objective standards ionising radiation is not a great cancer risk compared with other environmental and behavioural factors.
However, syllabuses tend to make unquantified assertions along the lines of 'State the effects of ionising nuclear radiations on living things, including cell death, mutations and cancer' without ever mentioning how incredibly rare and unlikely these outcomes are.
This is why I've tried to make a distinction between the rather low-res syllabus requirements and what the effects actually are.
Key animations from the video for you to use in front of a class
Effect of large doses
Alpha ionisation in cells
DNA damage
Natural vs. radiation DNA damage
Syllabus and real cancer risk
Alpha, beta and gamma risk
7.3 Managing risks
Short video summary (1:44)
-
There are three approaches to reducing radiation dose
- Putting something in the way - shielding
- Increasing the distance between people and the source
- Reducing the time spent being irradiated
-
We try to keep the radiation dose people get as low as reasonably possible. This is particularly relevant for people who work with radiation, for example technicians at hospitals and workers at nuclear power plants.
Since being around radioactive materials is part of their job, there are three approaches to reduce the dose from being irradiated by it: shielding, distance and time.
For example the thick glass in a 'hot cell' for working with radioactive materials provides shielding, and a remote controlled arm allows the technician to work with radioactive material at a distance. These health professionals have their schedules planned so they minimise the time they spend near the radioactive source in this cancer treatment machine.
The best way to stop being unnecessarily irradiated is to reduce contamination. To stop radioactive material ending up where it shouldn't be you ideally want to make sure it's a solid that's very difficult to dissolve in water.
Pieces of scrap metal from a nuclear power plant are set in cement, which keeps them in one place. If your radioactive source is temporarily a liquid or a gas then you need to make sure it's in a container that it can't escape from, for example when a radioactive tracer is injected into the patient.
If you're deliberately releasing it into the environment, for example as a medical or environmental tracer, then you want to make sure it has a short half-life, so it stops being radioactive fairly quickly.
-
Even though the risks from nuclear radiation are low, regulations tend to be written as if it was much more risky than it is. For example, even though there is no evidence for increased cancer risk above a dose of about 100 mSv, regulations assume that a dose of 1 mSv is twice as hazardous as a dose of 0.5 mSv, though in all likelihood the risk from both is zero.
This is why radioactive sources at school are surrounded by such extravagant safety procedures, completely disproportionate to the risk that they pose, but guaranteed to communicate that here is something uniquely dangerous.
Most of the evidence for cancer risk comes from cohort studies of survivors of the Hiroshima and Nagasaki atom bombs, who received a single, large, whole-body dose of neutron radiation. This single large dose is extrapolated back linearly as if it applied to cumulative small doses.
Key animations from the video for you to use in front of a class
Shielding round a hot cell
Summary of protection strategies
Protective clothing limits contamination
Shielding in gamma therapy
7.4 Risk perception
Short video summary (3:35)
-
Statistically nuclear radiation is comparatively low risk
Some people fear it because they misunderstand the science
Other people fear it for rational but not scientific reasons
-
People tend not to be worried about nuclear radiation used for medical imaging or for cancer treatment, even though the doses for cancer treatment are huge compared with background radiation (which also tends not to worry people) .
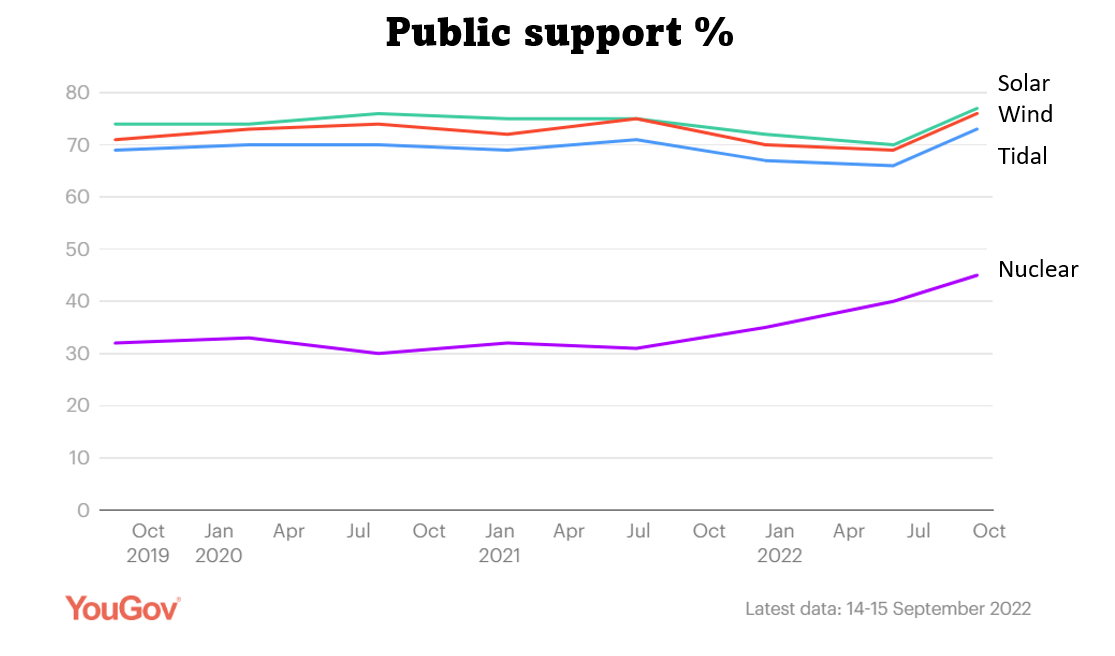
Nuclear power is only about half as popular as other low-carbon methods for generating electricity such as solar and wind. People who are against nuclear are mainly concerned about expense and safety, particularly nuclear accidents and nuclear waste.
The best scientific evidence is that the Chernobyl reactor meltdown will cause fewer than 200 deaths over a period of 60 years. The earthquake and subsequent tsunami that caused the meltdown at Fukushima in Japan killed at least 20,000 people, but with zero deaths from radiation, despite widespread contamination of the local area.
So even though Nuclear seems as safe as many other low-carbon methods for generating electricity it still has a bad rep. Here are some of the most common concerns about nuclear power:
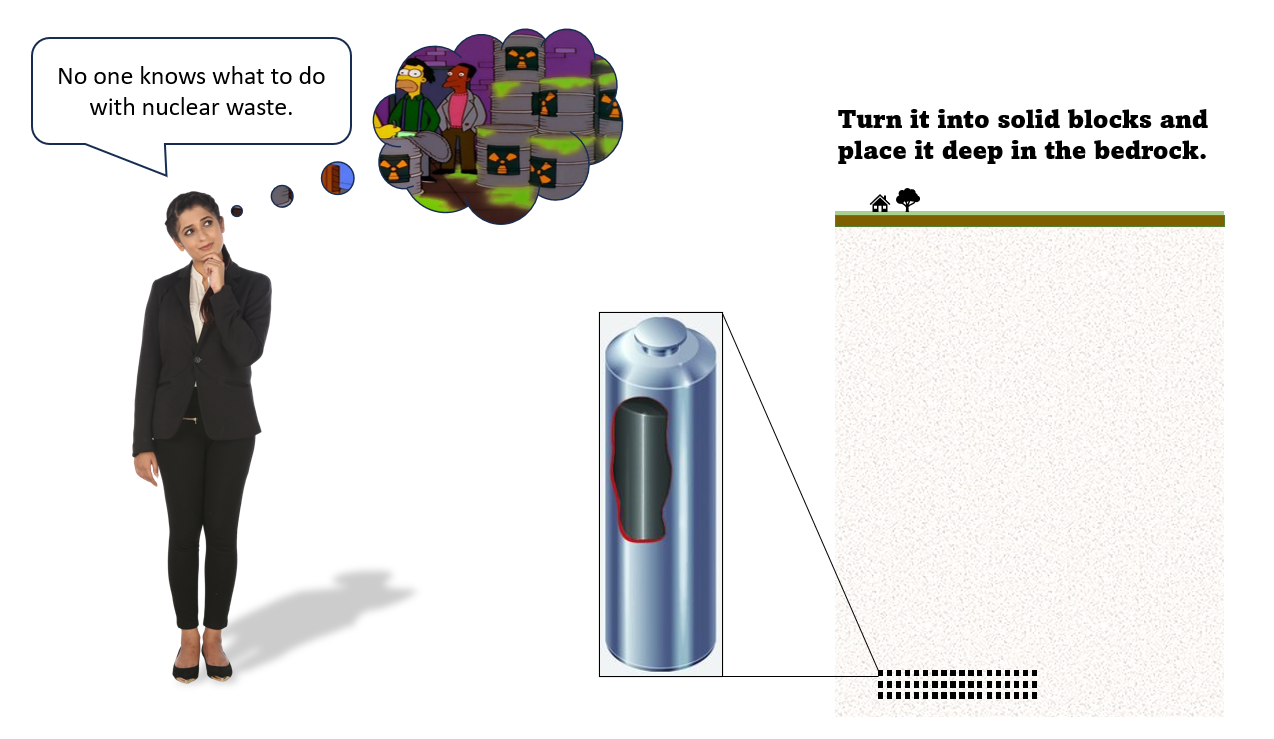
No one knows what to do with nuclear waste
To dispose of the most radioactive waste, it's made into solid blocks and placed in sealed engineered chambers deep in the bedrock. This keeps it away from people. To be extra safe the solid waste is encased in strong metal sleeves, and a site is chosen that's very dry and not subject to earthquakes.
The waste will be lethal for hundreds of thousands of years
As we've seen in lesson 5, very long half-life means very low radioactivity. The most hazardous isotopes have half-lives of about 30 years, since they're significantly radioactive for a human lifetime, but these lose 99.9% of their radioactivity within the first 300 years.
The waste containers will eventually fail, and the waste will leak out
High-level waste is always disposed of as a solid. The waste is like the green pigment in this glass - the green can't leak even if the glass is broken.
The radiation might still drift up to the surface
Remember our sparkler analogy. If the sparkler doesn't move, then the sparks can't travel any distance. In the same way radiation can't travel long distances - only waste - and that's a solid that doesn't dissolve in water.
But people don't use facts and reason when they decide whether to accept a risk or not - they use these three rules of thumb instead:
One - Who is offering me this risk? - Because if I think they're bad people then I'm not going to accept any risk, no matter how small
Two - What's the personality of the risk? If it's something that's scary, artificial, invisible and controlled by people I don't trust then I don't want to accept the risk.
Three - What precautions do I see people take? Why would you put waste hundreds of meters underground in incredibly strong containers, and talk about safety the whole time. It must be really dangerous.
These three rule of thumb are all 'rational' but they aren't really scientific. Where do you sit? Do you believe 'the science' or does it still just sound too worrying?
-
I used to work as a consultant working out how to communicate the risks of radioactive waste disposal. The accepted way to do this was - and still is - to point to all the safety measures. None of this makes radioactive waste disposal feel safe, merely that it's so dangerous that it requires incredibly careful management that is unprecedented for any other kind of waste.
I conducted the only informal research that I'm aware of of adult understanding of nuclear waste disposal with Professor Geraldine Thomas, who was at the time Professor of Molecular Pathology at Imperial College. What was interesting was that science graduates had just as many misconceptions about radioactive waste as non-scientists, but they were much more certain that their incorrect understanding was actually accurate!
When people worry about the risks from radiation based on the three heuristics of
Do I like the people offering me this risk?
How does the risk make me feel?
What do I see people doing to mitigate it?
they are being entirely rational, but simply not 'scientific' -in the sense of making some kind of dispassionate calculation of the product of likelihood and consquence.
Key animations from the video for you to use in front of a class
Public support for nuclear power
Cancer treatment vs. background dose
Chernobyl and Fukushima
Slides: Radioactivity misconceptions