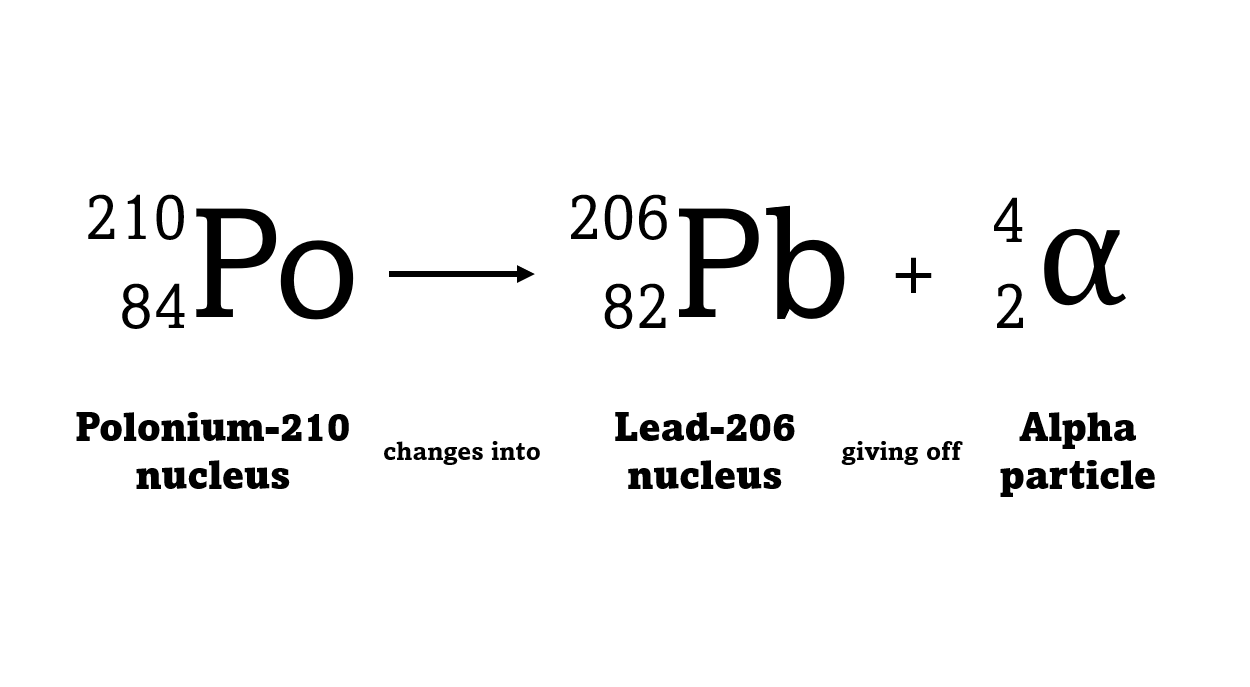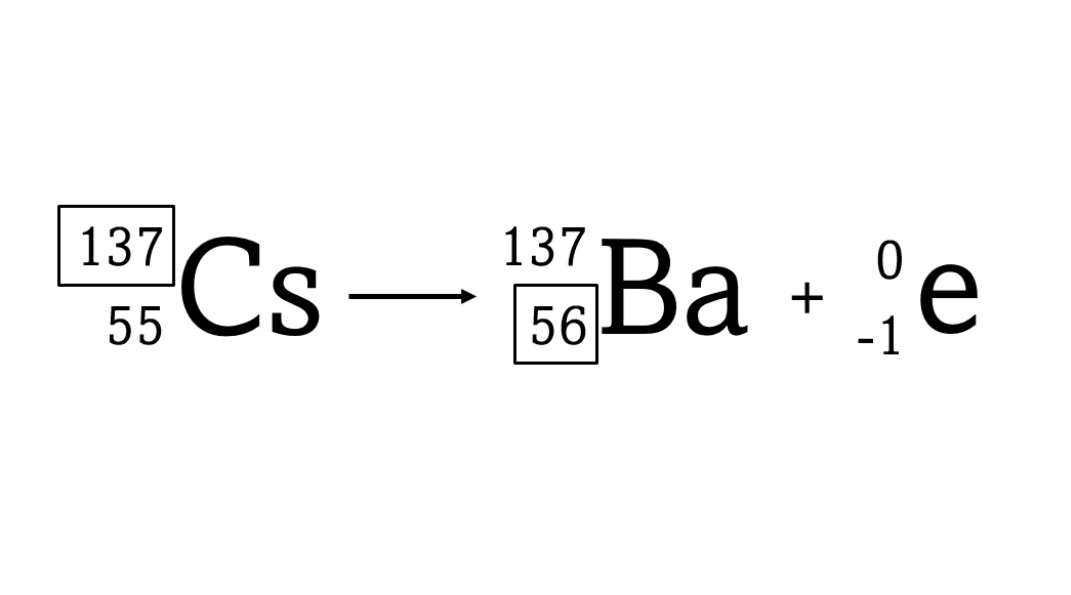Radioactivity Explained
8. Nuclear equations
New elements, same building blocks
8.1 Changing elements
8.2 Balancing equations
8.1 Changing elements
Short video summary (3:51)
-
A radioactive decay causes two events - the nucleus of an atom changes and an alpha beta or gamma is given off
Alpha and beta decay cause the element to change because the number of protons changes
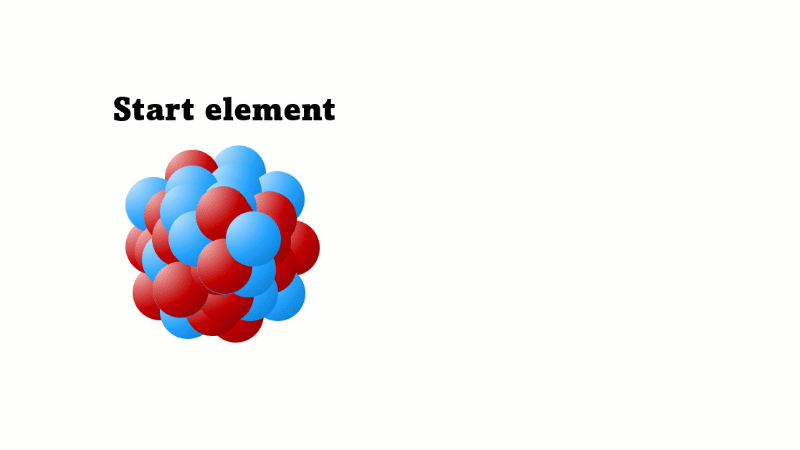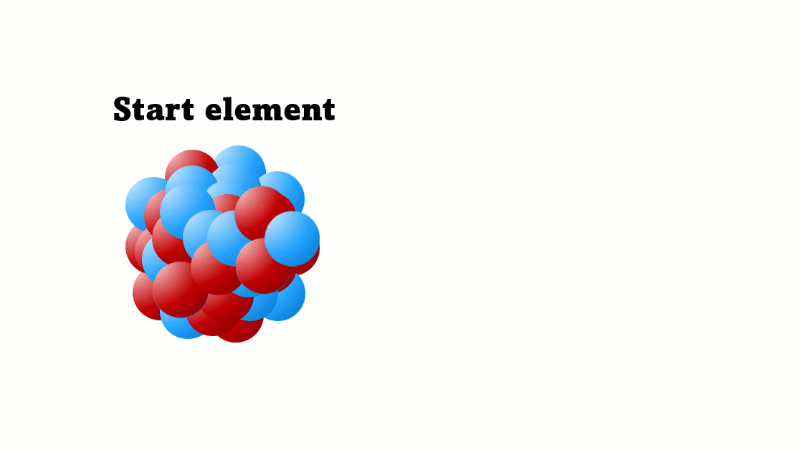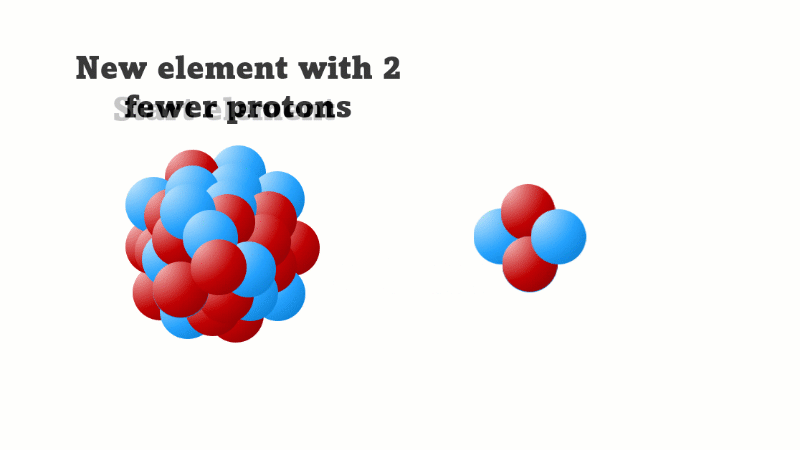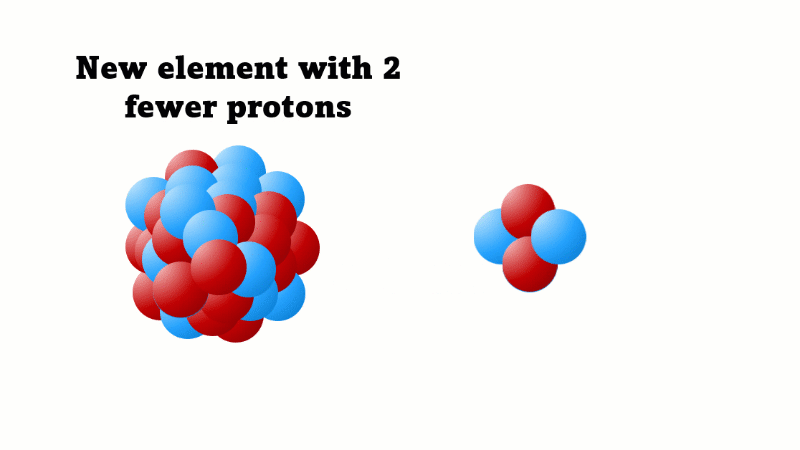
Alpha decay causes the nucleus to lose two protons (and two neutrons)
Beta decay causes the nucleus to gain a proton (because a neutron changes into a proton)
Gamma does not cause a change in element
-
A radioactive decay causes two events - the nucleus changes and at exactly the same time a particle is given off. We can describe a decay using a decay equation.
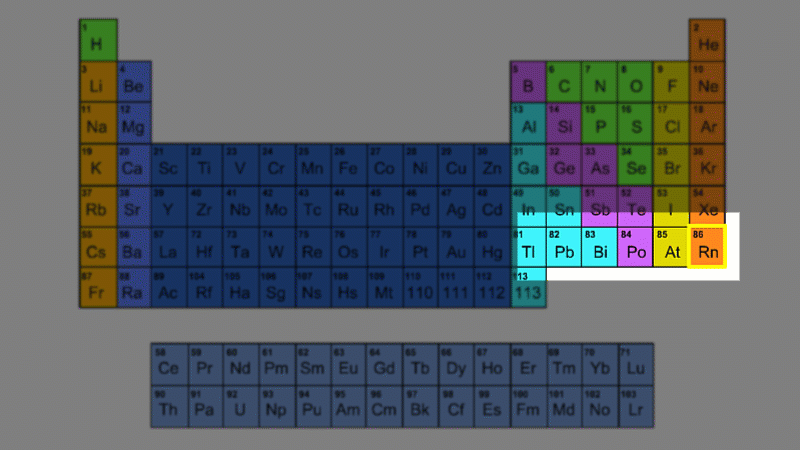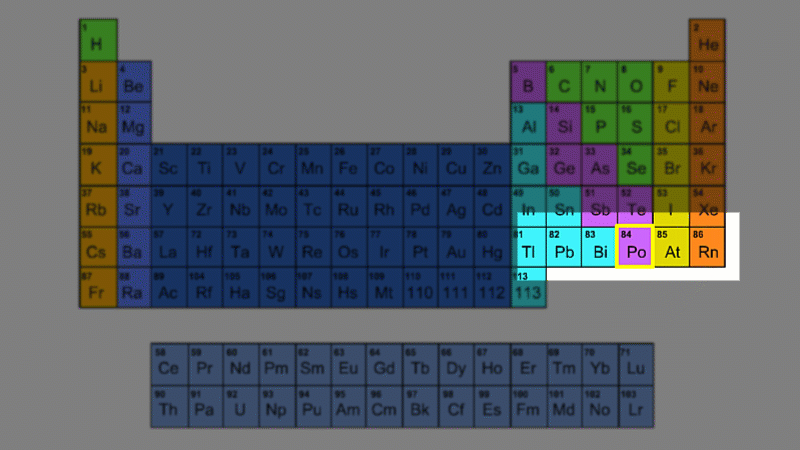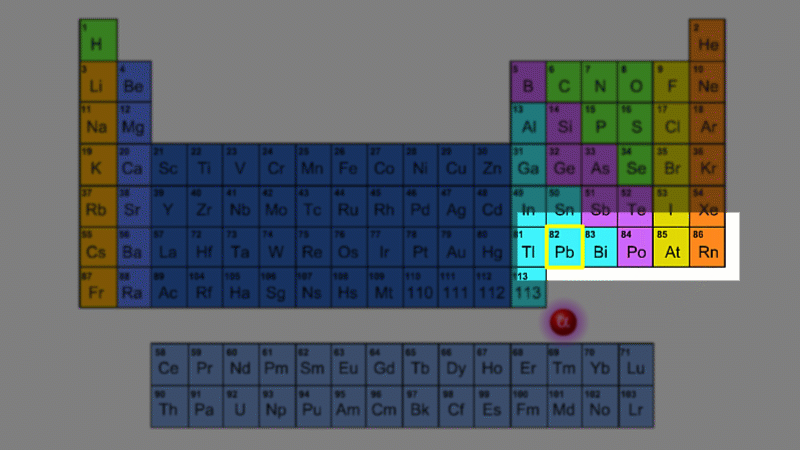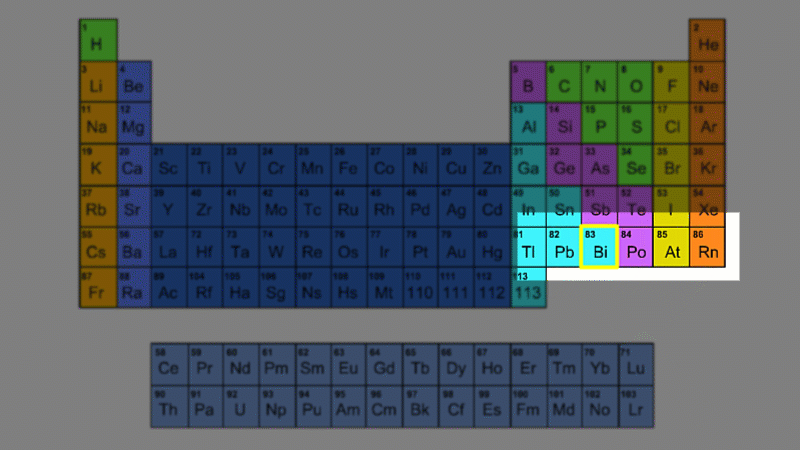
For example polonium-210 changes into lead-206 by giving off an alpha particle. We know this change is because the number of protons changes from 84 to 82, and it's the number of protons that defines an element.
The whole point with Dalton's atomic theory was that atoms couldn't just change from one element to another. But with the discovery of radioactivity it became clear that they did this all the time.
Let's go back to Rutherford's lab in 1902 - almost ten years before he figured out that atoms had a nucleus and therefore before the discovery of the proton. He was investigating the radioactivity of thorium and he noticed the radioactivity dropped suddenly when someone came into the room. His conclusion was that the thorium was actually turning into a new element which is a gas. This new element ultimately acquired the name 'radon'.
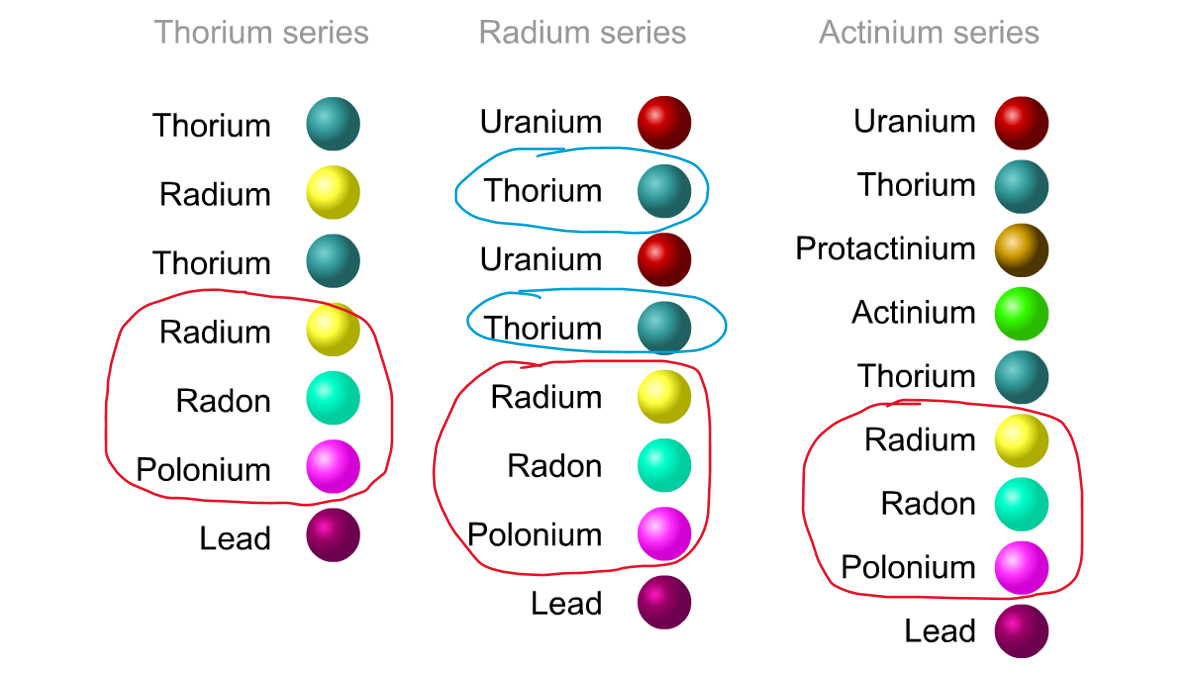
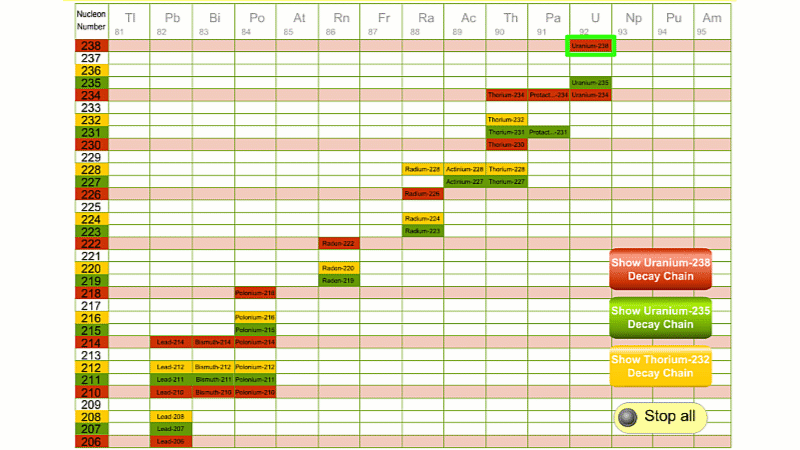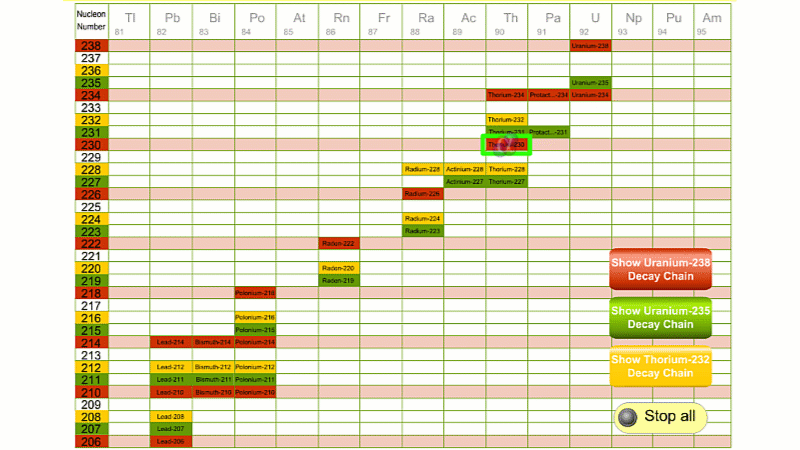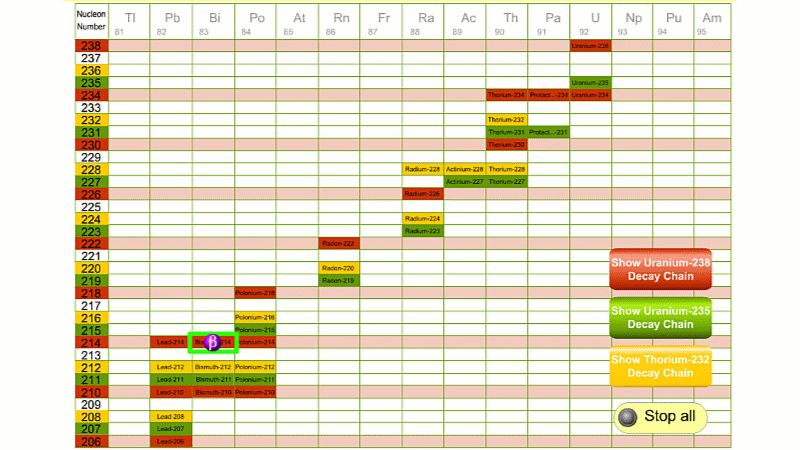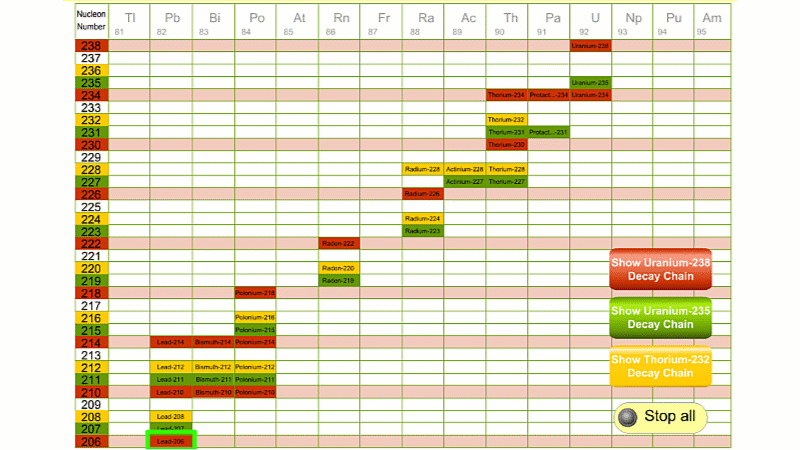
His colleague Frederick Soddy carried on investigating, and discovered that the nucleus of a radioactive atom often went through several decays before finally becoming stable. He found that there were three natural decay chains, each of which ended up with an isotope of lead. There were elements that were common to all three decay chains, for example radium, radon and polonium and sometimes the same element appeared twice in the same chain.
Even though the elements are the same, the isotope is different for each instance. We can now explain what was going on.
An alpha particle consists of 2 protons and 2 neutrons, so it has an atomic number of 2 and a mass number of 4. It's identical to a helium nucleus. When an alpha emitter decays, the new element has two fewer protons. So it changes to an element with an atomic number that's two lower in the periodic table.
A beta particle is just an electron. Electrons have a tiny mass - you need roughly 2000 of them to have the same mass as a proton. So we give them a mass number of zero. Because a beta particle is negatively charged we give it an atomic number of -1. When a beta emitter decays, the new element has one extra proton. So it changes to an element with an atomic number that one higher in the periodic table.
Gamma is just light - with no mass and no charge. So it has a mass number of zero and an atomic number of zero. Gamma decay leaves the number of protons and neutrons the same, but they are rearranged into a lower energy state.
Sometime a decay chain goes from alpha to alpha, decreasing mass and changing element each time. Sometimes after an alpha decay has reduced the atomic number by two, you get two beta decays. Each beta decay creates a new proton from a neutron. So the atomic number goes back up by two, and you end up back at the same element - just with an isotope of a lower atomic mass.
-
Nuclear equations are typically taught in a rather mechanical way, but I think it's important to emphasise how extraordinary was (and is) the idea that it's possible (indeed common) for the atom of one element to turn into the atom of another quite spontaneously.
This went against one of the key tenets of two thousand years of atomic theory - that atoms were immutable. When we hear clicks on our Geiger counter it's because at an invisibly small scale, alchemy is going on as one element changes into another.
Key animations from the video for you to use in front of a class
Individual atom showing multiple decays
Structure of a decay equation
Slideshow: Effects on nucleus
Decay and the periodic table
The three natural decay chains
Change in mass and atomic number
8.2 Balancing equations
Short video summary (2:29)
-
How to balance nuclear equations
Total mass number must be the same on each side of the equation
Total proton number must be the same on each side of the equation
-
In the previous section I tried to explain why nuclear equations were important, because they described how an atom changed element when its nucleus gave out an alpha or a beta particle.
In this section, we're going to look at balancing nuclear equations, without worrying too much about what they mean.
The mass number on the left must equal the total mass number on the right because the total number of protons and neutrons must stay the same.
And the atomic number on the left must equal the total atomic number on the right because the total electric charge must stay the same.
-
Though I've tried to give equations some context in the previous section, this is probably best treated as a purely mathematical exercise.
Just bear in mind that a nuclear equation is a description of how a nucleus changes. It's not saying that we have two separate nuclei that are equivalent to each other somehow.
Mass number (strictly nucleon number) is conserved because you don't gain or lose nucleons. A neutron may change into a proton during beta decay, but the total number of nucleons stays the same.
One question is why a beta particle has an atomic number of -1. For me it's simply a convention to make the equation balance, but you could also say that it's because balancing atomic number is a statement of the conservation of electric charge.
Key animations from the video for you to use in front of a class
Slideshow: Nuclear equations