Radioactivity Explained
9. Nuclear power
Low carbon but not renewable
9.1 Generating electricity
9.2 Fission and moderators
9.3 Chain reactions and control rods
9.4 Radioactive waste
9.5 Nuclear fusion
9.1 Generating electricity
Short video summary (3:02)
-
Electricity is generated by spinning coils of wire very fast near a very strong magnet
It doesn't matter if you spin the wire or spin the magnet
To cause the spinning you blast super-heated, high-pressure steam through a turbine
Hot metal rods in a nuclear reactor are used to boil water into the steam that blasts through the turbine
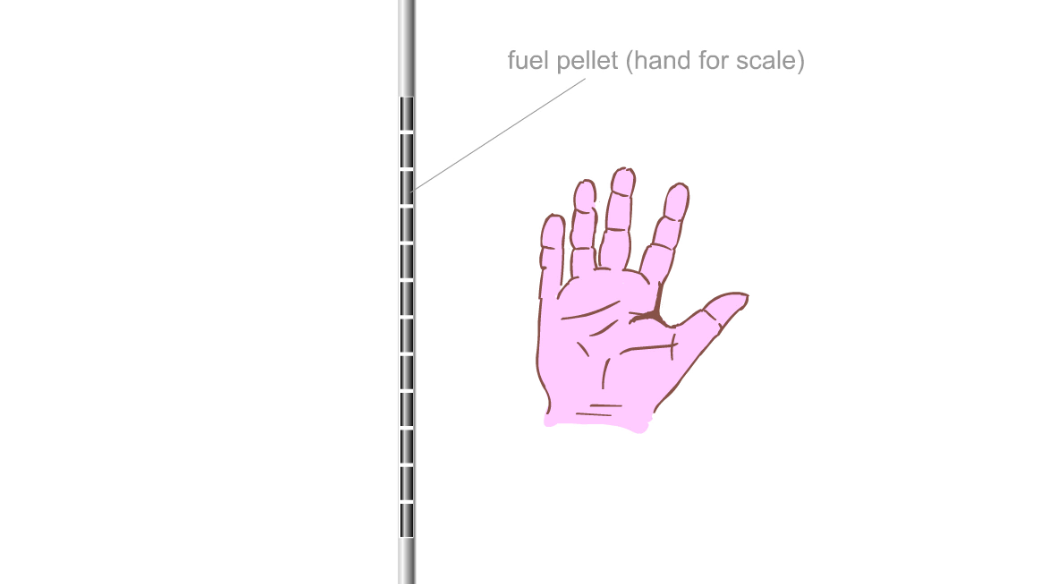
The hollow metal 'fuel rods' are filled with finger tip-sized uranium fuel pellets
It's the uranium fuel pellets where the nuclear reactions happen, and it's the fuel pellets that get hot
-
We can use the heat produced in a nuclear reactor to generate electricity. Let's work backwards.
The most common way to generate electricity is to spin coils of wire very fast near magnets. It doesn't matter if you spin the coils or spin the magnets - the effect is the same.
A voltage is produced across the ends of each coil. And indirectly it's this voltage that allows all our fridges, TVs and mobile phone chargers to work.
A big generator in a power station may support the electrical needs of 50,000 people. Think of trying to spin 50,000 washing machines all at the same time, and it gives you some idea of why the forces needed to keep the generator spinning are huge.
To cause the spinning, you blast super-heated high-pressure steam through a turbine. The turbine design is very complex, and you can't see it moving because it's enclosed in heavy casing to keep the steam in. The steam isn't like the clouds you see when you're cooking. It's pressurised to about 45 atmospheres, heated to 500 degrees celsius and screams through the turbine at upwards of 1000 km/h. Think of being near a big jet engine if you want an idea of the sensation.
To shift energy quickly enough to run all those electrical appliances you need to boil about a tonne of liquid water per second completely away into super-heated high-pressure steam. This requires a lot of heat.
Some power stations still use powdered coal, but it's much cleaner to use a nuclear reactor. A nuclear reactor in a power plant is like a big kettle heated by metal rods that get hot all by themselves. Inside the metal rods are little uranium fuel pellets. And it’s the nuclear reactions that happen in the fuel pellets that cause the heating. Each pellet can provide enough heat to generate electricity for a person for about a year.
Typically you pass high-pressure water through the reactor and use that water to turn the water that passes through the turbine into high pressure steam. This means the turbine side is isolated from the nuclear side. The water that passes through the reactor is sometimes called a 'coolant', but this name is a bit misleading. It's a throwback to when scientists built the first experimental reactors and they simply passed water through them to keep everything cool. In modern reactors the water isn't there to 'cool' the reactor but to do the crucial job of moving heat from the reactor core to the boiler.
Some reactors are designed to use carbon dioxide gas or molten metal to shift heat around, but the pressurised water reactor is by far the most common.
-
I personally like to work backwards from macroscopic ideas about how electricity is generated to sub-microscopic ideas about nuclear fission, not the other way round.
When we see (or imagine) something spinning we tend not to have much of a sense of just how difficult it is to spin. Riding a bicycle is a useful analogy. It's very easy to make the wheel spin if you lift it off the ground; it's harder if you're cycling over level ground, and much, much harder if you're cycling uphill. This is because the effort you need to make the wheel spin doesn't depend much on the wheel itself but on the useful work you get out of riding the bike (for example increasing your gravitational potential energy).
It's the same with a generator - how hard it is to turn doesn't depend much on the generator itself (even though it may be the size and weight of a car) but on what the generator is running. The more things the generator is running - in the thousands of homes, offices and factories - then the harder it is to turn. This is because the more things it runs, the bigger the current it has to provide, and this current creates its own huge magnetic field in the generator that always opposes the magnetic field making it turn.
The turning effect needed is huge - something like hanging a Range Rover on a lever 1 km long. The experience of being in a turbine hall where super-heated high-pressure steam is being blasted through the turbine to spin the generator is like being near the jets of an aeroplane operating at full power, and that's because that's where the energy needed to run the lives of thousands of people is concentrated in one place.
The nuclear reactor is a bit like a magic electric kettle where the heating element gets hot all by itself. To keep the nuclear reactions going you just need millions of fuel pellets arranged in the right way, and they just spontaneously get hot and stay hot - liberating heat quickly enough to cause the drama of the sound and fury in the turbine hall where the electricity is generated.
Key animations from the video for you to use in front of a class
Fuel pellets in a fuel rod
Generating electricity
A steam turbine
9.2 Fission and moderators
Short video summary (2:13)
-
Fission is when a nucleus breaks apart into two smaller nuclei
Fission doesn't normally happen spontaneously - you need to add a neutron to a large, already unstable nucleus
After a fission the two smaller nuclei created are moving very fast, and as they slow they heat the material they're in
A neutron needs to be going comparatively slowly to be absorbed by a nucleus and cause fission
A moderator is a material that slows down fast neutrons enough to cause fission
Water is the most common moderator, but some older reactors use a type of carbon called graphite
-
In the previous section we saw how the metal fuel rods in a nuclear reactor get hot because of nuclear reactions in the uranium fuel pellets. The kind of nuclear reaction is called fission.
This is when an atom with a very large, fairly unstable nucleus like uranium splits into two smaller atoms. Even though it's atoms that split, we're only going to worry about the nucleus.
The two smaller nuclei move off at very high speed, but quickly come to rest. And their lost kinetic energy appears as thermal energy in the fuel pellet. In other words, the fissions make the fuel pellets hot.
Actually the process is a little more complex than this. Radioactivity is random and spontaneous. You can't tell when it will happen and you can't speed it up or slow it down.
Fission can happen spontaneously but in a nuclear reactor you make it happen in a controlled way. You do this by making the nucleus absorb another neutron. It's then too big to be stable and breaks into two large-ish parts and a small number of neutrons.
It's these neutrons that can go on to cause further fissions in what's called a chain reaction.
The neutrons that are released in fission are generally going too fast for them to be absorbed by nearby nuclei. So to get a chain reaction you need to slow them down and get them to cause a fission in a neighbouring fuel rod.
You slow down the neutrons by placing a material called a moderator between the fuel rods. In a pressurised water reactor the moderator is just the water that the fuel rods are heating up. The hydrogen atoms in the water are good at slowing neutrons down because they have roughly the same mass.
Some reactor designs from the 1970s and 80s use graphite as a moderator, but these are coming to the end of their lives. One advantage of using water as a moderator is that if you lose the water then the fission reactions rapidly stop.
-
We've deliberately not gone sub-microscopic in the previous lesson. We've just said 'nuclear reactions' make the fuel pellets hot. In this lesson we dig deeper into what these nuclear reactions actually are, and how we make them happen.
You can make any nucleus fission if you try hard enough, but it's only useful as a way to generate heat if the nucleus is so big that it's very near the point of breaking apart spontaneously. The extra neutron it absorbs just pushes it over the stability boundary and it splits.
Fission is not about smashing nuclei into pieces but coaxing them to accepting that 'wafer-thin mint' of an extra neutron. And that only works if the neutron is going at what are known as 'thermal' speeds, in other words of the order of the random thermal motion of the particles that make up the materials.
We don't talk in depth about neutrons released during fission causing further fissions until the next lesson when we cover chain reactions, but this is mentioned in the context of moderators. The neutrons released during fission tend to be moving very fast - about 10% of light speed, say ~10^7 m/s - whereas neutrons need to be travelling at ~10^3 m/s to have a good chance of being absorbed by another nucleus.
The moderator - normally water - is between fuel rods, so a fission by a fuel pellet in one fuel rod tends to produce a fission in a pellet in a different fuel rod, rather than a nearby nucleus in the same fuel pellet, as we tend to show it in our visualisations.
Because only very large nuclei can usefully be made to fission, they also tend to be long half-life alpha emitters, but this radioactivity is irrelevant to the actual fission process. It's worth emphasising this point - radioactivity is a spontaneous random process that we can't change the rate of, but fission is a deliberate process that requires a lot of planning, engineering and continuous control to happen.
Key animations from the video for you to use in front of a class
Fast neutrons don’t cause fission
Fission - Breaking apart a big nucleus
Fission producing heat
Slow ‘thermal’ neutrons cause fission
Neutron slowed by water moderator
Neutron slowed by hydrogen atom
9.3 Chain reactions and control rods
Short video summary (1:25)
-
When a big nucleus fissions some free neutrons are released
These free neutrons can go on to cause further fissions in a 'chain reaction'
In a nuclear reactor the chain reaction is controlled using control rods
The control rods fit between the fuel rods and absorb neutrons
-
You start a nuclear reactor by introducing a neutron source, which starts the chain reaction, as a neutron from one fission causes the next one.
We've shown the nuclei reforming after they fission, which obviously they don't do, but it keeps the simulation tidy. Just imagine that each time they reform we're focusing on a different nucleus. We've also simplified it by not showing the moderator.
After a while we can remove the neutron source and the reaction sustains itself because each fission releases neutrons that cause on average exactly one other fission. When this balance is reached the reactor is said to be critical.
If we want to reduce the power of the reactor, in other words reduce the number of fissions per second then we lower the control rods. These are made of a material that absorbs neutrons (for example boron), and stop the nuclei near the top of the fuel rods taking part in the chain reaction.
If we lower them all the way down then we can shut down the reactor and the fission reactions stop.
-
A uranium fuel pellet can generate heat through fission for several years, but they get less and less efficient at doing this over time, as the fissionable uranium gets used up.
For this reason the control rods are gradually raised to keep the reaction rate constant. The reactor needs to be shut down every 18 months or so for refuelling. This involves replacing about a third of the fuel rods each time, and typically takes several weeks.
To shut the reactor down in an emergency the control rods are dropped under gravity into the reactor, and nuclear fission will stop in a matter of seconds. This is called a reactor SCRAM. There is a probably apocryphal story that SCRAM stands for Safety Control Rod Axe Man because in very early experimental reactors the control rods were suspended over the reactor by a rope which could be cut in an emergency.
Key animations from the video for you to use in front of a class
Shutting down the reactor
Starting the reactor with a neutron source
Reducing reactor power
9.4 Radioactive waste
Short video summary (2:17)
-
Uranium fuel pellets are hardly radioactive when they are new
They are highly radioactive when they come out of the reactor
Fission creates radioactive isotopes
Radioactive waste can be safely disposed of because it's a solid and placed out of the way deep in the bedrock
-
Fresh uranium fuel pellets are made from two isotopes of uranium. Both have half-lives of over a billion years so they're hardly radioactive at all. As they undergo nuclear reactions over a period of a year or so they get more and more radioactive.
When uranium nuclei fission into two smaller nuclei (plus some neutrons), one nucleus is typically a bit bigger than the other. These two smaller nuclei - called fission products - typically have too many neutrons to be stable. So they are beta emitters - achieving stability by changing one or more of their neutrons into protons. Many are gamma emitters, too. Most, though not all, tend to have shorter half-lives from hours up to a 100 years or so.
As well as splitting in half, a uranium nucleus sometimes absorbs one or more neutrons without splitting. This creates new elements with nuclei bigger than uranium. They're called transuranic elements - because they're beyond uranium in the periodic table. They have cool names like plutonium, neptunium and americium.
Transuranic elements are all radioactive and because their nuclei are too large to be stable, they are typically alpha emitters. They tend to have long half-lives, from a few decades to millions of years.
All these waste atoms - the small nuclei fission products and the big-nucleus transuranics - just remain in the fuel pellet. When a fuel pellet comes out of a reactor, about 95% of it is just the same as when it went in - unchanged uranium. The fuel pellets themselves don’t look any different, but the short half-life fission products make them highly radioactive.
They are a ceramic, so are very hard, and dissolve in water at about the same rate as granite. This is the main reason why it’s safe to dispose of them deep in the bedrock - the radiation itself only travels a very short distance, and the radioactive material just stays in one place away from people. There’s more about the risks of long half-life waste in lesson 7.
-
Most syllabuses mention the radioactive waste produced by nuclear power without any real detail.
In this lesson I try to briefly outline the origins of the waste and how it can be safely disposed of. I haven't mentioned half-life here because it seemed to belong more in the lessons on half-life and uses and dangers. It is worth reiterating that the most hazardous half-lives are of the order of decades, not the tens of thousands of years that the press obsess about. The longer the half-life, the lower the radioactivity.
One level of detail that I haven't gone into too much, but is worth bearing in mind, is that there are two uranium isotopes present in fuel pellets. About 96% is uranium-238. This doesn't fission, and almost all comes out of the reactor exactly the same as when it went in, but it does absorb neutrons to create the transuranic elements, particularly plutonium-239, which happens to about 1% of the U-238. It's the ~4% uranium-235 that does the fissioning, and that's where the radioactive fission products originate.
U-235 makes up only about 0.7% of natural uranium so centrifuges are used to separate it from its slightly more massive sibling, U-238. This process is called enrichment. For power reactors the fuel is enriched to ~4%. The same process can be continued to get to the 90% enrichment you need to make a bomb. This is why enrichment technology is so carefully controlled.
Key animations from the video for you to use in front of a class
Why the isotopes are radioactive
Fresh fuel pellets are hardly radioactive
Fission creates radioactive isotopes
New radioactive elements
The amount of waste is tiny
Disposing of radioactive waste
9.5 Nuclear fusion
Short video summary (2:32)
-
Nuclear fusion involves temperatures and pressures that are normally only found at the centre of stars
It involves smashing small nuclei like hydrogen together to make a bigger nucleus like helium
It is very difficult because the electrostatic repulsion is so big
No one knows if we will ever generate electricity from fusion at scale, but it is unlikely to happen before 2050
Fusion involves readily available fuel - e.g. hydrogen from water - and leaves very little radioactive waste
-
All current nuclear power plants work by harnessing the heating effect of nuclear fission in uranium fuel pellets. It's a mature technology that's been generating electricity since 1956 in over 400 sites around the world. It operates at temperatures and pressures that are not dissimilar to gas or coal power plants and the engineering is well understood.
Fusion is completely different - it's like trying to create the conditions at the centre of the Sun on Earth. Even though scientists have been working on fusion since the 1950s, as of 2023 they've still only managed to create sustained fusion for under 20 minutes.
Fission is all about splitting a big nucleus like uranium into smaller ones - this isn't too difficult, since a uranium nucleus is so big that it's on the verge of breaking apart anyway. Fusion involves trying to convince two or more small nuclei - typically hydrogen in other words protons, or one of its isotopes - to join together and form a larger nucleus, typically helium.
This is very, very difficult because the electrostatic repulsion is so large. They have to get sufficiently close for the strong nuclear force to take effect and bind the nuclei together. Even at the centre of the Sun with its huge pressures and temperatures, fusion between two nearby hydrogen nuclei is very rare. But because the Sun is so huge that still means there's enough fusion to create all the heat and light that we experience on Earth.
If you want protons to get close enough together to fuse then you need very high speeds, and that means very high temperatures - millions of degrees Celsius. Creating these temperatures and pressures is what makes fusion so expensive and technically difficult.
When you do get nuclei close enough to fuse, very large amounts of energy is given off - about 10 times as much energy per kilogram as for fission. When two protons fuse together, the resulting nucleus has a slightly lower mass than the two protons when they were separate.
If fusion could ever be made to work at scale then it could generate large amounts of electricity with very little environmental impact. The products of fission - mostly helium - are not radioactive, so there isn't the waste challenge that fission reactors face.
-
Fission and fusion are inevitably introduced at a sub-microscopic level first. I think it might be better to differentiate their macroscopic characteristics first, and then go sub-microscopic.
So to answer the question what's the difference between fission and fusion, I'd be inclined to start with the idea that fission involves temperatures and pressures that are common in a jet engine, whereas for fusion you're trying to create the conditions at the centre of a star.
That's why fission is a common and well understood way to generate electricity all over the world, whereas as of the end of 2023 the best scientists had only managed to establish self-sustaining fusion for less than 20 minutes, never mind develop some viable way of generating electricity from the process.
Key animations from the video for you to use in front of a class
Fusion needs huge temperatures
Fusion is the process that runs stars
Fusion liberates more energy per kg
Fused nuclei have a slightly lower mass